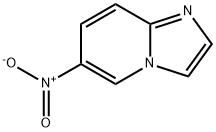
6-NITROIMIDAZO[1,2-A]PYRIDINE synthesis
- Product Name:6-NITROIMIDAZO[1,2-A]PYRIDINE
- CAS Number:25045-82-3
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
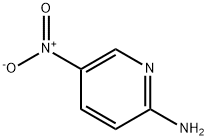
4214-76-0
516 suppliers
$6.00/10g
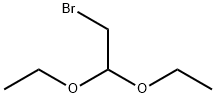
2032-35-1
480 suppliers
$5.00/10g
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
106 suppliers
$7.00/250mg
Yield:25045-82-3 96.7%
Reaction Conditions:
Stage #1: Bromoacetaldehyde diethyl acetalwith hydrogenchloride in water at 48; for 2 h;
Stage #2: 5-nitro-pyridin-2-ylaminewith sodium carbonate in water at 65; pH=8 - 9; for 2 h;
Steps:
2.2.1. Synthesis of N-(8-iodoimidazo[1,2-a]pyridin-6-yl)acetamide ( 6 )
H 2 O (100 mL), bromoacetaldehyde diethyl acetal (97%, 108.09 mL, 696.96 mmol), and hydrochloric acid (10 mL) were added to a clean round-bottom flask (250 mL). The mixture was heated to 48 °C, and then cooled to room temperature by stirring for 2 h. The pH was adjusted to 8-9 with solid Na 2 CO 3 in an ice water bath, and compound 2 (50 g, 359.43 mmol) was added. Next, the reaction mixture was heated to 65 °C for 2 h. The reactional time was monitored by TLC using mixture of petroleum ether and ethyl acetate (1:1) and the plates were revealed with sublimated iodine supported on silica gel. At the end of the reaction, the mixture was cooled to room temperature and the pH of the mixture was ad- justed to 9-10 by adding 10% NaOH solution. After the adjustment, a solid was precipitated out, and was then filtered and dried under vacuum to afford the yellow powder 3 (56.70 g, yield 96.70%).
References:
Chen, Dongmei;Chen, Yumei;Liao, Weike;Wu, Qingmei;Zhang, Xiaohan;Zhou, Zhixu [Journal of Molecular Structure,2021,vol. 1245]
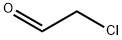
107-20-0
4 suppliers
$22.60/5ml

4214-76-0
516 suppliers
$6.00/10g
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
106 suppliers
$7.00/250mg

4214-76-0
516 suppliers
$6.00/10g
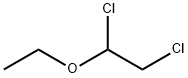
623-46-1
79 suppliers
$329.00/25g
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
106 suppliers
$7.00/250mg