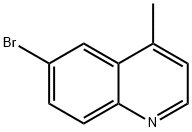
6-Bromo-4-methylquinoline synthesis
- Product Name:6-Bromo-4-methylquinoline
- CAS Number:41037-28-9
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
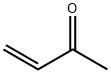
78-94-4
4 suppliers
$29.69/25ml
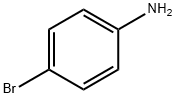
106-40-1
471 suppliers
$12.00/5g

41037-28-9
95 suppliers
$12.00/100mg
Yield: 81%
Reaction Conditions:
Stage #1:4-bromo-aniline with hydrogenchloride;chloranil in ethanol;water at 20; for 0.0833333 h;
Stage #2:methyl vinyl ketone in ethanol;water at 75; for 4.5 h;
Steps:
1.1 6-bromo-4-methylquinoline
To a stirred solution of 4-bromoaniline (20 g, 1 17 mmol) and chloranil (34.7 g, 141 mmol, 1.2 equiv.) in ethanol (200 ml_, 10 volumes) at rt was added 37% HCI (29.3 ml_, 351 mmol, 3 equiv.) over five minutes. After stirring for five minutes at room temperature the reaction mixture was heated to 75°C where methyl vinyl ketone (15.45 ml_, 190.5 mmol, 1.5 equiv.) diluted in ethanol (15 ml_, 1 volume) was added to the reaction mixture over thirty minutes. After four hours, no starting material was observed by TLC. The reaction was cooled to 60°C where it was diluted with THF (165 ml_, 1 1 volumes), stirred for an hour at 60°C and then left to cool to room temperature overnight. The suspension was filtered through a sintered Buchner funnel. The residue was rinsed with THF (2 x 45 ml_, 6 volumes in total) and then dried at 50°C overnight in a vacuum oven to give the title compound (21.1 g, 81 %) as a brown solid. (0243) 1H NMR (400 MHz, CDCI3) d 8.78 (d, J = 4.4 Hz, 1 H), 8.16 - 8.14 (m, 1 H), 7.97 (d, J = 9.0 Hz, 1 H), 7.77 (dd, J = 9.0, 2.2 Hz, 1 H), 7.25 (dd, J = 4.4, 0.9 Hz, 1 H), 2.68 (d, J = 0.9 Hz, 3H). (0244) 13C NMR (101 MHz, DMSO) d 145.76, 138.20, 135.96, 133.04, 129.21 , 127.79, 125.12, 123.25, 122.18, 19.24.
References:
FUNDACIó INSTITUT DE RECERCA BIOMèDICA (IRB BARCELONA);INSTITUCIó CATALANA DE RECERCA I ESTUDIS AVAN?ATS;UNIVERSITAT DE BARCELONA;TAURIELLO, Daniele;BYROM, Daniel;MATARíN MORALES, Joan Antoni;BATLLE GóMEZ, Eduard;RIERA ESCALé, Antoni WO2020/104648, 2020, A2 Location in patent:Page/Page column 46