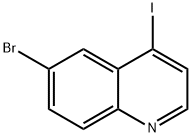
6-BROMO-4-IODOQUINOLINE synthesis
- Product Name:6-BROMO-4-IODOQUINOLINE
- CAS Number:927801-23-8
- Molecular formula:C9H5BrIN
- Molecular Weight:333.95
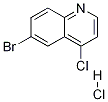
1086062-75-0
1 suppliers
inquiry

927801-23-8
74 suppliers
$32.00/250mg
Yield: 94%
Reaction Conditions:
with potassium iodide in acetonitrile for 48 h;Reflux;
Steps:
9 4.1.9. 6-Bromo-4-iodoquinoline (12)
4.1.9
6-Bromo-4-iodoquinoline (12)
To a solution of 6-bromo-4-chloroquinoline (11) (3.50 g, 14.46 mmol) in anhydrous EtOAc (20 mL) was added HCl-saturated EtOAc (40 mL) and a white precipitate formed immediately.
After stirring for 30 min, the suspension was concentrated under vacuum to afford 6-bromo-4-chloroquinoline hydrochloride as an off white solid (3.91 g, 14.14 mmol). A two-neck flask was charged with 6-bromo-4-chloroquinoline hydrochloride (3.91 g, 14.14 mmol), anhydrous potassium iodide (9.76 g, 70.70 mmol) and anhydrous acetonitrile (100 mL).
The resulting slurry was stirred at reflux for 48 h and allowed to cool to room temperature.
Saturated aqueous NaHCO3 solution (40 mL) was added to the mixture, followed by 20 mL of a 5% sodium sulfite solution.
The reaction mixture was extracted with CH2Cl2 (200 mL * 2).
The combined organic extracts were dried over magnesium sulfate and concentrated in vacuo to give the crude product, which was further purified by silica gel column chromatography (25% ethyl acetate/petroleum ether) to give the title compound (4.42 g, 13.27 mmol, 94% yield) as an off-white solid. 1H NMR (500 MHz, DMSO-d6) δ 8.51 (d, J = 4.5 Hz, 1H, Ar-H), 8.21 (d, J = 4.5 Hz, 1H, Ar-H), 8.11 (t, J = 1.5 Hz, 1H, Ar-H), 7.97-7.91 (m, 2H, Ar-H). ESI-MS: m/z = 334 [M+H]+.
References:
Lv, Xiaoqing;Ying, Huazhou;Ma, Xiaodong;Qiu, Ni;Wu, Peng;Yang, Bo;Hu, Yongzhou [European Journal of Medicinal Chemistry,2015,vol. 99,art. no. 7904,p. 36 - 50]

65340-70-7
266 suppliers
$7.00/250mg

927801-23-8
74 suppliers
$32.00/250mg
![5-[(4-Bromophenylamino)methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione ,98%](/CAS2/GIF/187278-01-9.gif)
187278-01-9
19 suppliers
$110.00/500mg

927801-23-8
74 suppliers
$32.00/250mg
106-40-1
469 suppliers
$12.00/5g

927801-23-8
74 suppliers
$32.00/250mg

145369-94-4
217 suppliers
$10.00/250mg

927801-23-8
74 suppliers
$32.00/250mg