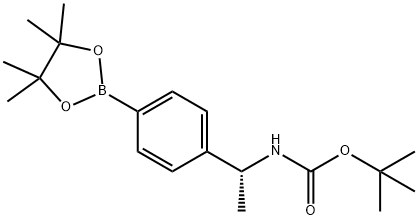
(R)-tert-butyl 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan- synthesis
- Product Name:(R)-tert-butyl 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-
- CAS Number:578729-05-2
- Molecular formula:C19H30BNO4
- Molecular Weight:347.26
![(R)-[1-(4-Bromophenyl)ethyl]carbamic acid tert-butyl ester](/CAS/GIF/578729-21-2.gif)
578729-21-2
87 suppliers
$30.00/100mg
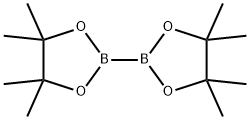
73183-34-3
570 suppliers
$6.00/5g

578729-05-2
28 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride at 80; for 1 h;
Steps:
III
To a solution of the above carbamate (7.6 g, 25.3 mmol) in DMSO (20 mL) was added bis (pinacolato) diboron (7.07 g, 27.9 mmol), dichloro [l, l'-bis (diphenylphosphino) ferrocene] palladium (II) dichloromethane adduct (2.06 g, 2.53 mmol), and potassium acetate (7.45 g, 76.0 mmol) at room temperature under N2. The resulting mixture was heated at 80 °C for 1 hour. The reaction was quenched by addition of EtOAc and filtered through celite. The organic extract was washed with water three times, saturated NaCl, dried over MgSO4, filtered and concentrated under vacuum. The residue was purified on silica gel eluted with 0-20% ethyl acetate in hexane to provide tert-butyl (lR)-l- [4- (4, 4,5, 5-tetramethyl- 1, 3,2-dioxaborolan-2-yl) phenyl] ethylcarbamate as a clear light yellow oil with a mass ion (ES+) of 333
References:
WO2005/63690,2005,A1 Location in patent:Page/Page column 28
24424-99-5
833 suppliers
$13.50/25G

578729-05-2
28 suppliers
inquiry
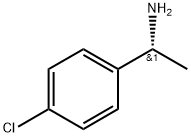
27298-99-3
93 suppliers
$19.00/100mg

578729-05-2
28 suppliers
inquiry