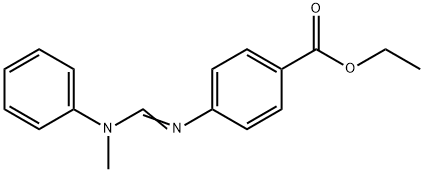
Ethyl 4-[[(methylphenylamino)methylene]amino]benzoate synthesis
- Product Name:Ethyl 4-[[(methylphenylamino)methylene]amino]benzoate
- CAS Number:57834-33-0
- Molecular formula:C17H18N2O2
- Molecular Weight:282.34

165 g (1 mol) of ethyl p-aminobenzoate, 117.7 g (1.1 mol) of N-methylaniline, 10 g of p-toluenesulfonic acid,200mL with toluene toluene added to the 1000mL reaction flask, stirred and warmed to 50-60 ° C, 2h dropwise 59.53g (1.1mol) formic acid(Content of 85%),Heating to reflux reaction, continue to separate the generated water, the reaction of about 5h to stop water when there is no water. The reaction solution was distilled off with atmospheric toluene recovery agent, toluene was recovered, the remaining vacuum distillation reaction solution,A pale yellow viscous liquid N- (4-ethoxycarbonylphenyl) -N'-methyl-N'-phenyl formamidine 262g, yield 93%, purity 99.4%.

94-09-7
777 suppliers
$5.00/50mg

100-61-8
389 suppliers
$8.00/1g
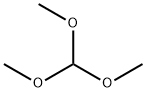
149-73-5
530 suppliers
$12.00/5g
![Ethyl 4-[[(methylphenylamino)methylene]amino]benzoate](/CAS/GIF/57834-33-0.gif)
57834-33-0
226 suppliers
$9.00/10g
Yield:57834-33-0 98%
Reaction Conditions:
with acetic acid in Petroleum ether at 50 - 65; for 2.25 h;Catalytic behavior;Green chemistry;Temperature;
Steps:
1-4; 1-3 A process for synthesizing N-(ethoxycarbonylphenyl)-N'-methyl-N'-phenylamidine, comprising the steps of:
1) Accurately weigh 16.5 g of ethyl p-aminobenzoate, 16.5 g of trimethyl orthoformate, 16.8 g of N-methylaniline into a 150 mL three-necked flask, and add 60 mL of petroleum ether to a three-necked flask and place it in magnetic stirring. A dropping funnel, a water separator and a thermometer were placed on the three-necked flask, and the outer wall of the water separator was pre-passed with a freezing liquid of -5 ° C. A three-necked flask was placed in a magnetic stirrer. 2) Stir and raise the temperature to a temperature of 50 ° C in a three-necked flask .9 g of glacial acetic acid was added dropwise to the reaction vessel within 15 min, and the reaction was kept for 0.6 h; The temperature was raised to 50 ° C, the reaction was 0.8 h; the temperature was further raised to 65 ° C, and the reaction was 0.6 h; The liquid in the lower layer of the water separator is continuously separated, and the reflux is stopped at the upper end of the water separator. 3) The reaction mixture was desolvated under a vacuum, then added 60 mL of toluene, cooled to 40 ° C; 4) Washing 3 times with hydrochloric acid aqueous solution of pH=2, using 50 mL of hydrochloric acid aqueous solution each time, washing for 8 min each time; then washing with 0.6 mol/L sodium bicarbonate aqueous solution 30 mL, adjusting pH to 8; finally washing 3 times with distilled water Every time you use40 mL of distilled water. 5) The organic layer after the water washing treatment was transferred to a rotary steaming flask for vacuum distillation, and the temperature of the vacuum distillation was controlled at about 125 ° C, and the product was purified by distillation under reduced pressure, the yield was 98%, and the chroma value was 26 Hazen.
References:
CN108640857,2018,A Location in patent:Paragraph 0027; 0033-0083

64-18-6
1085 suppliers
$22.12/250ML

94-09-7
777 suppliers
$5.00/50mg

100-61-8
389 suppliers
$8.00/1g
![Ethyl 4-[[(methylphenylamino)methylene]amino]benzoate](/CAS/GIF/57834-33-0.gif)
57834-33-0
226 suppliers
$9.00/10g
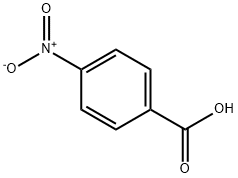
62-23-7
693 suppliers
$14.00/25g
![Ethyl 4-[[(methylphenylamino)methylene]amino]benzoate](/CAS/GIF/57834-33-0.gif)
57834-33-0
226 suppliers
$9.00/10g