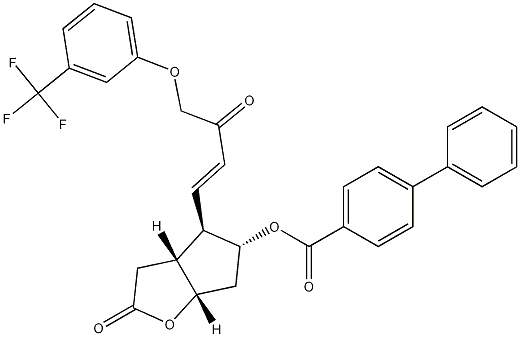
[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester synthesis
- Product Name:[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester
- CAS Number:54142-64-2
- Molecular formula:C31H25F3O6
- Molecular Weight:550.52
Yield:54142-64-2 91%
Reaction Conditions:
Stage #1: dimethyl [3-(3-(trifluoromethyl)phenoxy)-2-oxopropyl]phosphonatewith potassium carbonate in isopropyl alcohol at 25; for 0.5 h;Horner-Wadsworth-Emmons Olefination;
Stage #2: (3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl biphenyl-4-carboxylate in isopropyl alcohol at 25; for 3 h;Horner-Wadsworth-Emmons Olefination;Reagent/catalyst;Solvent;Temperature;
Steps:
6 Example 6: Manufacture of (3aR,4R,5R,6aS)-2-oxo-4-((E)-3-oxo-4-(3-(trifluoromethyl)phenoxy)but-1-enyl)hexahydro-2Hcyclopenta[b]furan-5-yl biphenyl-4-carboxylate.
Dimethyl 3-(3-(trifluoromethyl) phenoxy) -2-oxopropylphosphonate (0.46 g, 1.43 mmol, 1 eq) was added to a 100 ml 3-neck flask and 10 ml of IPA was added and stirred. K2CO3 (0.6 g, 4.29 mmol, 3 eq) was added at 25 ° C and stirred for 30 minutes. Coriolactone aldehyde (0.5 g, 1.43 mmol, 1 eq) was added and stirred at 25 ° C for 3 hours. The reaction was warmed to 25 ° C and stirred for 2 hours (TLC confirmed completion of the reaction, EA: Hx = 1: 1, rf = 0.65). The reaction was concentrated under reduced pressure (bath 40 ° C). To the reaction were added 10 ml of water and 10 ml of MC and the layers were separated. The water layer was added with 10 ml of MC and extracted one more time. NaCl aqueous solution was added thereto, followed by washing with Na2SO4, followed by filtration and concentration under reduced pressure to obtain a liquid compound.- To obtain 0.61g (yield: 91%). 1 H NMR (400MHz, CDCl3) δ (ppm): 8.04 (d, 2H, HAr), 7.58 (dd, 4H, HAr), 7.43-7.47 (m, 2H, HAr), 7.35-7.40 (m , 2H, HAr), 7.22 (s, 1H, HAr), 7.11 (s, 1H, HAr), 7.01-7.03 (m, 1H, HAr), 6.89 (dd, 1H, CH = CH), 6.56 (d, 1H, CH = CH), 5.33 (q, 1H, CH), 5.10 (t, 1H, CH), 4.72 (s, 2H, CH2), 2.85-2.97 (m, 3H, CH CH2), 2.31-2.65 ( m, 3H, CH2 CH).
References:
KR2016/15100,2016,A Location in patent:Paragraph 0096-0100
31752-99-5
245 suppliers
$19.00/250mg
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate](/CAS/GIF/54094-19-8.gif)
54094-19-8
130 suppliers
$65.00/1g
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/54142-64-2.gif)
54142-64-2
55 suppliers
inquiry

31752-99-5
245 suppliers
$19.00/250mg
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/54142-64-2.gif)
54142-64-2
55 suppliers
inquiry
![7,7-Dichlorobicyclo[3.2.0]hept-2-en-6-one](/CAS/GIF/5307-99-3.gif)
5307-99-3
75 suppliers
$47.89/5g
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/54142-64-2.gif)
54142-64-2
55 suppliers
inquiry
![2H-Cyclopenta[b]furan-2-one, 3,3-dichloro-3,3a,6,6a-tetrahydro-, (3aR,6aS)-](/CAS/20200611/GIF/87422-39-7.gif)
87422-39-7
1 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/54142-64-2.gif)
54142-64-2
55 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-4-formylhexahydro-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/38754-71-1.gif)