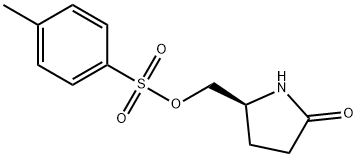
(S)-(+)-5-(Hydroxymethyl)-2-pyrrolidinone p-toluenesulfonate synthesis
- Product Name:(S)-(+)-5-(Hydroxymethyl)-2-pyrrolidinone p-toluenesulfonate
- CAS Number:51693-17-5
- Molecular formula:C12H15NO4S
- Molecular Weight:269.32
32503-27-8
631 suppliers
$5.00/25g
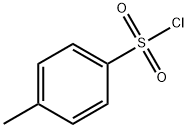
98-59-9
593 suppliers
$9.00/5g
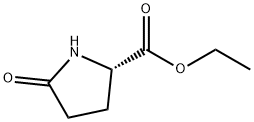
7149-65-7
238 suppliers
$15.00/25G

51693-17-5
72 suppliers
$12.00/250mg
Yield:-
Reaction Conditions:
with sodium borohydrid in dichloromethane;water;acetone
Steps:
9 (S)-5-Tosyloxymethyl-2-pyrrolidinone
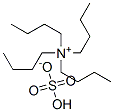
EXAMPLE 9 (S)-5-Tosyloxymethyl-2-pyrrolidinone Ethyl (L)-pyroglutamate (15.7 g), dissolved in water (50 ml) is added slowly at 0° C. to a solution of sodium borohydride (2.2 g) in water (50 ml). The mixture is allowed to warm up over a one-hour period after which it is stirred at room temperature for 20 minutes. Acetone (5 ml) is added and the stirring is continued for 30 minutes. Solvent is evaporated to give a dry residue which is dissolved in water (100 ml). The solution is then concentrated to a volume of 40 ml. To the concentrated solution are added: caustic soda (5 g), tosyl chloride (18.10 g) in dichloromethane (100 ml), and tetra-n-butyl ammonium hydrogen sulfate (1.03 g). The resulting mixture is stirred vigorously for 42 hours at room temperature. The organic layer is separated, and the aqueous layer is extracted with dichloromethane (50 ml). The organic layers are combined and dried. Evaporation of solvent gives a residue which is recrystallized from toluene (150 ml) to give 13.6 g of pure (S)-5-tosyloxymethyl-2-pyrrolidinone, [α]D =+7.80+-0.04 (c=2.64, EtOH).
References:
Merrell Dow Pharmaceuticals Inc. US4621145, 1986, A

98-59-9
593 suppliers
$9.00/5g
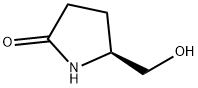
17342-08-4
332 suppliers
$5.00/1g

51693-17-5
72 suppliers
$12.00/250mg
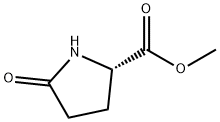
4931-66-2
278 suppliers
$10.00/5g

51693-17-5
72 suppliers
$12.00/250mg
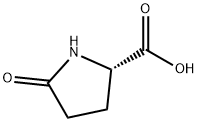
98-79-3
621 suppliers
$5.00/25g

51693-17-5
72 suppliers
$12.00/250mg