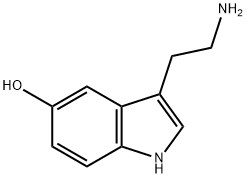
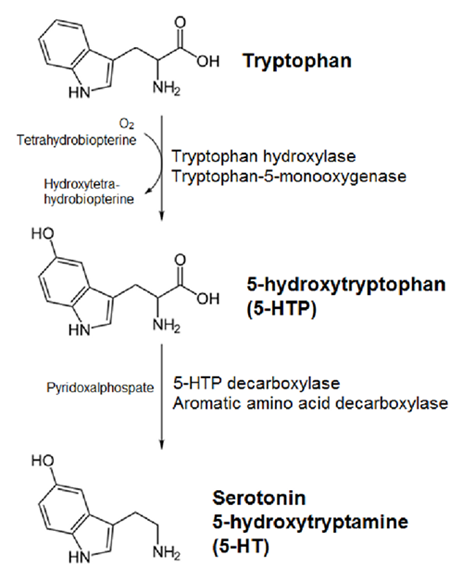
5-Hydroxytryptamine synthesis
- Product Name:5-Hydroxytryptamine
- CAS Number:50-67-9
- Molecular formula:C10H12N2O
- Molecular Weight:176.22
147918-24-9
8 suppliers
$650.00/100 mg

50-67-9
164 suppliers
$93.29/25mg
Yield:50-67-9 100%
Reaction Conditions:
with palladium 10% on activated carbon;ammonium formate in methanol at 70; for 0.75 h;Inert atmosphere;
Steps:
4.2.3 RRN 82,83,84General procedure for the hydrogenolysis for the synthesis of the natural products
General procedure: Psilocin, Bufotenin and Serotonin. To a stirred suspension of the suitable tryptamine (0.25mmol) and an equal weight of 10% Pd-C in dry methanol (7mL), anhydrous ammonium formate (71mg, 1.125mmol) was added in a single portion under N2. The resulting reaction mixture was stirred at 70°C for 45min. The solution was filtered through a pad of Celite, the solvent was removed under reduce pressure and the residue was purified by flash column chromatography on silica gel (DCM:MeOH 9:1 and 1% of TEA) to give the corresponding natural product in quantitative yield.
References:
Bartolucci, Silvia;Mari, Michele;Di Gregorio, Giovanni;Piersanti, Giovanni [Tetrahedron,2016,vol. 72,# 18,p. 2233 - 2238]
608-07-1
454 suppliers
$13.00/5g

50-67-9
164 suppliers
$93.29/25mg

4350-09-8
439 suppliers
$5.00/100mg

50-67-9
164 suppliers
$93.29/25mg

56-69-9
430 suppliers
$25.00/10g

50-67-9
164 suppliers
$93.29/25mg

111-76-2
751 suppliers
$9.00/1g

50-67-9
164 suppliers
$93.29/25mg