
5-FLUORO-1H-INDOLE-7-CARBALDEHYDE synthesis
- Product Name:5-FLUORO-1H-INDOLE-7-CARBALDEHYDE
- CAS Number:603306-52-1
- Molecular formula:C9H6FNO
- Molecular Weight:163.15
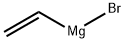
1826-67-1
212 suppliers
$60.89/100ml

603306-58-7
2 suppliers
inquiry

603306-52-1
44 suppliers
inquiry
Yield:603306-52-1 58.8%
Reaction Conditions:
Stage #1: vinyl magnesium bromide;2-(dibutoxymethyl)-4-fluoro-1-nitrobenzene in tetrahydrofuran at -40; for 1 h;
Stage #2: with hydrogenchloride in water at 20; for 1 h;
Steps:
Synthesis of Intermediate 6.
To a solution of intermediate 5 (50 g, 0.17 mol) in dry THF (1500 ml) was added vinylmagnesium bromide solution (1 M, 668.8 ml, 668.8 mmol) dropwise at -40° C. The mixture was stirred at -40° C. for 1 hr. TLC (petroleum ether/EtOAc=5/1) showed the reaction was completed Then the mixture was poured into aq. NH4Cl, extracted with EtOAc (300 ml×3), the organic phases were concentrated to give crude compound. The crude compound was purified by flash column chromatography (eluted with petroleum ether/EtOAc from 100:1 to 20:1) to give the compound (24 g) as yellow oil. To a solution of the compound (24 g) in THF (100 ml) was added HCl (0.5 N, 80 ml) dropwise at room temperature. The mixture was stirred at room temperature for 1 hr. TLC (petroleum ether/EtOAc=5/1) showed the reaction was completed. The mixture was adjusted PH=10 with aq. NaOH, extracted with EtOAc (300 ml×3), concentrated in vacuum to give intermediate 6 (16 g, 58.8%) as yellow solid. (0338) 1H NMR (CDCl3, 400 MHz): δ (ppm) 10.06 (s, 1H), 7.62 (dd, 1H, J=2 Hz, 9.2 Hz), 7.38-7.41 (m, 2H), 6.60 (t, 1H, J=2.4 Hz).
References:
US2018/214458,2018,A1 Location in patent:Paragraph 0336-0338

1021910-38-2
1 suppliers
inquiry

603306-52-1
44 suppliers
inquiry
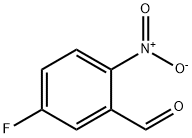
395-81-3
209 suppliers
$10.00/5g

603306-52-1
44 suppliers
inquiry
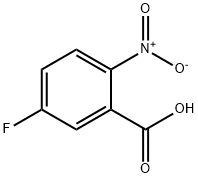
320-98-9
288 suppliers
$8.00/5g

603306-52-1
44 suppliers
inquiry
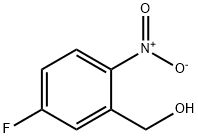
287121-32-8
44 suppliers
inquiry

603306-52-1
44 suppliers
inquiry