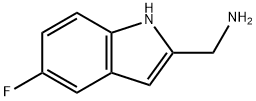
(5-FLUORO-1H-INDOL-2-YL)METHANAMINE synthesis
- Product Name:(5-FLUORO-1H-INDOL-2-YL)METHANAMINE
- CAS Number:883531-07-5
- Molecular formula:C9H9FN2
- Molecular Weight:164.18
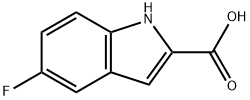
399-76-8
234 suppliers
$12.00/1g

883531-07-5
25 suppliers
$161.65/250mg
Yield:883531-07-5 76%
Reaction Conditions:
Stage #1: 5-fluoroindole-2-carboxylic acidwith 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Stage #2: with ammonium hydroxide in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Stage #3: with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 2 h;Reflux;Inert atmosphere;
Steps:
3 Synthesis and Characterization of (5-fluoro-1H-indol-2-yl)methanamine
To a stirred solution of 5-Fluoroindole-2-carboxylic acid 14 (5.00 g, 27.8 mmol) in THF (150 mL) was added CDI (9.00 g, 55.8 mmol). The reaction mixture was stined for 2 h before the addition of NH4OH (28-30% aq. solution, 50 mL). The reaction mixture was then stirred for 2 h before being quenched with H2O (100 mL). The layers were separated and the aqueous phase was extracted with EtOAc (2 c 100 mL). The combined organic extracts were washed with brine, dried (MgS04) and concentrated in vacuo. The crude residue was dissolved in THF (150 mL) and cooled to 0 °C. LiAlH4 (2.50 g, 65.9 mmol) was added slowly and the reaction mixture was warmed to RT before heating to reflux and stirred for 2 h. The reaction mixture was then quenched with H2O (100 mL). The layers were separated and the aqueous phase was extracted with EtOAc (2 x 100 mL). The combined organic extracts were washed with brine, dried (MgS04) and concentrated in vacuo. Purification by column chromatography (MeOH 10% NH4OH / CH2CI2, 0:→1 1:9 gradient run) provided the amine 12 (3.45 g, 21.0 mmol, 76%) as a white solid.1H NMR (DMSO-d6, 300 MHz) d 11.7 (br s, 1H), 7.29 (dd, J= 8.8, 4.5 Hz, 1H), 7.18 (d, J= 10.1, 1.3 Hz, 1H), 6.83 (dt, .7= 9.2, 1.7 Hz, 1H), 6.23 (s, 1H), 3.84 (s, 2H); 13C NMR (DMSO-d6, 75 MHz) d 156.8 (d, J (C, F) = 229.9 Hz), 144.6, 132.7, 128.4 (d, J (C, F) = 9.9 Hz), 111.6 (d, J (C, F) = 9.5 Hz), 108.0 (d, J(C, F) = 25.8 Hz), 104.0 (d, J (C, F) = 23.2 Hz), 97.9 (d, J (C, F) = 3.2 Hz), 48.4.
References:
WO2019/136558,2019,A1 Location in patent:Paragraph 00104; 00139; 00147-00149
462068-77-5
5 suppliers
inquiry

883531-07-5
25 suppliers
$161.65/250mg