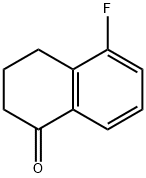
5-Fluoro-1-tetralone synthesis
- Product Name:5-Fluoro-1-tetralone
- CAS Number:93742-85-9
- Molecular formula:C10H9FO
- Molecular Weight:164.18
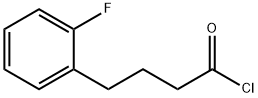
166251-26-9
1 suppliers
inquiry
143654-62-0
24 suppliers
$66.00/100mg

93742-85-9
93 suppliers
$65.00/100mg
Yield: 84.2 g (93%)
Reaction Conditions:
with hydrogenchloride;thionyl chloride;aluminium trichloride in carbon disulfide
Steps:
12.e e)
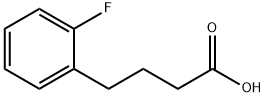
e) Preparation of 5-Fluorotetralone A mixture of 4-(2-fluorophenyl)butyric acid (100 g, 0.55 mol) and thionyl chloride (418.8 g, 3.51 mol) was refluxed for 3 h. The excess thionyl chloride was removed in vacuo to give 110.1 g (100%) of 4-(2-fluorophenyl)butyryl chloride. To the 4-(2-fluorophenyl)butyryl chloride in carbon disulfide (1.0 L) at -78° C. was added aluminum chloride (93.2 g, 0.7 mol) portionwise over a 30 min period. The mixture was warmed to room temperature for 30 min, then refluxed for 2 h. The reaction mixture was poured into a mixture of ice (500 mL) and HCl (6N, 500 mL). The carbon disulfide layer was separated, washed with saturated sodium bicarbonate and extracted with ethyl acetate. The aqueous phase was extracted with ethyl acetate. The combined extracts were dried (MgSO4) and concentrated in vacuo to give 84.2 g (93%) of 5-fluorotetralone as a tan solid. NMR (CDCl3): d 7.7 (m, 1H, ArH), 7.1 (m, 2H, ArH), 2.9 (t, 2H, CH2), 2.6 (t, 2H, CH2), 2.1 (q, 2H, CH2).
References:
Glaxo Wellcome Inc. US6124284, 2000, A

143654-62-0
24 suppliers
$66.00/100mg

93742-85-9
93 suppliers
$65.00/100mg

166251-26-9
1 suppliers
inquiry

93742-85-9
93 suppliers
$65.00/100mg
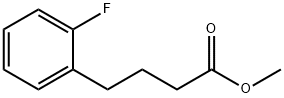
911825-55-3
0 suppliers
inquiry

93742-85-9
93 suppliers
$65.00/100mg
348-52-7
292 suppliers
$6.00/5g

93742-85-9
93 suppliers
$65.00/100mg