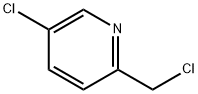
5-CHLORO-2-(CHLOROMETHYL)PYRIDINE synthesis
- Product Name:5-CHLORO-2-(CHLOROMETHYL)PYRIDINE
- CAS Number:10177-24-9
- Molecular formula:C6H5Cl2N
- Molecular Weight:162.02
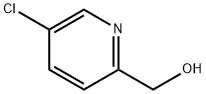
209526-98-7
119 suppliers
$17.00/100mg

10177-24-9
85 suppliers
$45.00/50mg
Yield: 89%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 4 h;
Steps:
5-chloro-2(chloromethyl)pyridine; [00372] To a 0 °C solution of 5-chloropicolinic acid (3.00 g, 19.0 mmol) indichloromethane (20 mL) was added sulfurous dichloride (2.78 mL, 38.1 mmol), after which the reaction was warmed to room temperature and stirred at that temperature for 4 hours. The reaction was then concentrated to dryness, then reconstituted in dichloromethane (5 mL). Methanol (10 mL) was added to the reaction mixture, and the reaction was allowed to stir at room temperature for 12 hours, after which it was then diluted with water, extracted with ethyl acetate (3 x 50 mL), washed with water, then washed with saturated sodium bicarbonate solution, dried (magnesium sulfate), filtered and concentrated. Purification was achieved by silica gel chromatography (ISCO 80g) using 0 to 80% ethyl acetate in hexanes to afford methyl 5-chloropicolinate was as an off-white solid (2.75 g, 15.2 mmol, 80% yield ).[00373] To a 0 °C solution of methyl 5-chloropicolinate (2.70 g, 15.7 mmol) in methanol (50 mL) was added sodium borohydride (1.79 g, 47.2 mmol), after which the reaction was warmed to room temperature and stirred at that temperature for 4 hours. The reaction mixture was then concentrated to a residue which was treated with 1M hydrochloric acid solution (15 mL), extracted with ethyl acetate (3 x 100 mL), washed with water, then washed with saturated sodium bicarbonate solution, dried (magnesium sulfate), filtered and concentrated. Purification was achieved by silica gel chromatography (ISCO 80g) using 0 to 60% ethyl acetate in hexanes to afford (5-chloropyridin-2-yl)methanol was as an off-white solid (2.15 g, 14.9 mmol, 95% yield).[00374] To a 0 °C solution of (5-chloropyridin-2-yl)methanol (2.10 g, 14.6 mmol) in dichloromethane (10 mL) was added sulfurous dichloride (1.60 mL, 21.9 mmol) followed by N,N-dimethylformamide (50 μ), after which the reaction was warmed to room temperature and stirred at that temperature for 4 hours. The reaction mixture was then concentrated to a residue which was reconstituted in water (15 mL), ethyl acetate (15 mL), and saturated sodium bicarbonate solution (15 mL). The organic layers were separated and washed with saturated sodium chloride solution, dried (magnesium sulfate), filtered and concentrated. Purification was achieved by silica gel chromatography (ISCO 40g) using 0 to 50% ethyl acetate in hexanes to afford 5-chloro-2(chloromethyl)pyridine as an light brown oil (2.11 g, 13.0 mmol, 89% yield).
References:
IRONWOOD PHARMACEUTICALS, INC.;HUDSON, Colleen;BARDEN, Timothy, C.;JIA, James;MERMERIAN, Ara;PENG, Bo;YANG, Jane;YU, Xiang, Y.;SPROTT, Kevin WO2012/88469, 2012, A1 Location in patent:Page/Page column 152-153
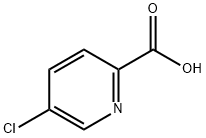
86873-60-1
245 suppliers
$7.00/1g

10177-24-9
85 suppliers
$45.00/50mg
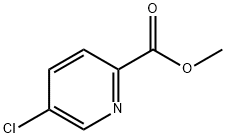
132308-19-1
158 suppliers
$6.00/250mg

10177-24-9
85 suppliers
$45.00/50mg
31181-73-4
0 suppliers
inquiry

10177-24-9
85 suppliers
$45.00/50mg
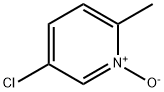
52313-58-3
11 suppliers
inquiry

10177-24-9
85 suppliers
$45.00/50mg