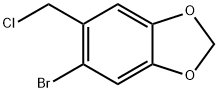
5-BROMO-6-(CHLOROMETHYL)-1,3-BENZODIOXOLE synthesis
- Product Name:5-BROMO-6-(CHLOROMETHYL)-1,3-BENZODIOXOLE
- CAS Number:64603-67-4
- Molecular formula:C8H6BrClO2
- Molecular Weight:249.49

20850-43-5
159 suppliers
$17.67/1gm:

64603-67-4
17 suppliers
inquiry
Yield:64603-67-4 99%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20; for 13 h;Inert atmosphere;
Steps:
10.1 Preparation of 5-bromo-6- (chloromethyl) benzo [d] [1,3] dioxole
In step (1) of Example 9 under an argon atmosphereThe resulting 5- (chloromethyl) benzo [d] [1,3] dioxole (2.29 g, 13.42 mmol) was dissolved in acetonitrile (27 mL), and N-bromosuccinimide (2.39 g, 16.11 mmol ) Was added. The reaction solution was stirred at room temperature for 13 hours, and the completion of the reaction was confirmed by TLC (hexane: ethyl acetate = 2: 1). After the reaction mixture was poured into water, the organic solvent layer extracted with dichloromethane (10 mL x 3) was washed again with saturated sodium chloride solution (50 mL), and the organic solvent layer was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to remove the solvent . The concentrate was separated and purified by column chromatography (hexane: ethyl acetate = 5: 1) to obtain the desired compound (3.32 g, 99% yield).
References:
KR2016/59366,2016,A Location in patent:Paragraph 0303; 0304; 0305; 0306
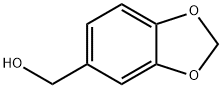
495-76-1
289 suppliers
$15.00/5g

64603-67-4
17 suppliers
inquiry
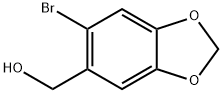
6642-34-8
55 suppliers
$38.00/100mg

64603-67-4
17 suppliers
inquiry
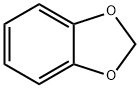
274-09-9
477 suppliers
$5.00/5g

64603-67-4
17 suppliers
inquiry
15930-53-7
151 suppliers
$5.00/1g

64603-67-4
17 suppliers
inquiry
