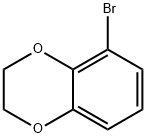
5-BROMO-2,3-DIHYDRO-1,4-BENZODIOXANE synthesis
- Product Name:5-BROMO-2,3-DIHYDRO-1,4-BENZODIOXANE
- CAS Number:58328-39-5
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
14381-51-2
154 suppliers
$11.00/1g

106-93-4
395 suppliers
$15.00/5g

58328-39-5
76 suppliers
$36.00/100mg
Yield: 47%
Reaction Conditions:
with potassium fluoride;potassium carbonate in N,N-dimethyl-formamide at 135; for 2 h;Inert atmosphere;
Steps:
9 Synthesis of 4-[2-[2-(2,3-di hydro-1 ,4-benzodioxine-5-sulfonamido)- phenyl]ethynyl]benzoic acid
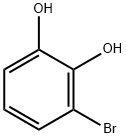
(Note:“-0” stands for“-OH”) In a 100-mL round-bottom flask, purged and maintained with an inert atmosphere of nitrogen, 3-bromobenzene-1 ,2-diol (3 g, 15.08 mmol, 1 eq, 95%) and 1 ,2-dibromoethane (4.5 g, 22.76 mmol, 1.5 eq, 95%) were dissolved in N,N-dimethylformamide (60 ml_). Potassium carbonate (4.5 g, 30.93 mmol, 2.1 eq, 95%) and KF (462 mg, 7.55 mmol, 0.5 eq, 95%) were added. The resulting mixture was stirred for 2 h at 135°C. After completion of the reaction the mixture was cooled to room temperature and the solids were filtered out. The liquid phase was washed with 4x20 ml_ of H20, dried over anhydrous sodium sulfate, filtered and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 :50). This resulted in 1.81 g (47%) of 5-bromo-2,3-dihydro-1 ,4- benzodioxine as colorless oil.
References:
MERCK PATENT GMBH;RYVU THERAPEUTICS S.A.;HEINRICH, Timo;PETERSSON, Carl;KRIER, Mireille;GONDELA, Andrzej;GALEZOWSKI, Michal Mikolaj;FABRITIUS, Charles-Henry Robert Yves;NOWAK, Mateusz Oktawian;KROL, Marcin WO2020/127960, 2020, A1 Location in patent:Page/Page column 132