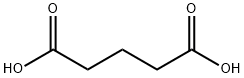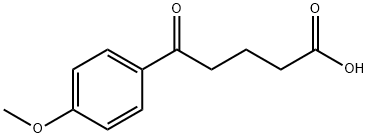
5-(4-methoxyphenyl)-5-oxopentanoic acid synthesis
- Product Name:5-(4-methoxyphenyl)-5-oxopentanoic acid
- CAS Number:4609-10-3
- Molecular formula:C12H14O4
- Molecular Weight:222.24
Yield:4609-10-3 96%
Reaction Conditions:
with hydrogenchloride;aluminum (III) chloride in dichloromethane;water at 10; for 0.2 h;Friedel-Crafts Acylation;
Steps:
6.1; 6.2; 6.3; 6.4 Example 6:
(1) Glutaric anhydride (compound 2) and anisole (compound 1) are uniformly mixed to obtain a homogeneous A solution. The concentration of glutaric anhydride (compound 2) in the homogeneous A solution is 1.5 mol/L. (2) Anhydrous aluminum trichloride, anisole (compound 1) and dichloromethane are mixed uniformly to obtain a homogeneous B solution. The concentration of aluminum trichloride in the homogeneous B solution is 0.75 mol/L. The molar ratio of aluminum oxide to anisole (compound 1) is 1:1.7. (3) Dilute concentrated hydrochloric acid with water to make C solution, the concentration of hydrochloric acid in C solution is 1mol/L. (4) The first micro-reaction mixer M1 and the first reaction module L1 are both placed at a preset temperature, and the homogeneous A solution obtained in step (1) and the homogeneous B solution obtained in step (2) are combined The first advection pump P1 and the second advection pump P2 are respectively pumped into the first micro-reaction mixer M1 according to the flow rate ratio (1:4), and the first advection pump P1 pumps the above homogeneous A solution into the first micro-reaction mixing In the vessel M1, the flow rate is 6 mL/min, and the mixture is uniformly mixed and transferred to the first reaction module L1 (inner diameter: 3 mm, length: 9 m) for Friedel-Crafts acylation reaction, the reaction temperature is 10° C., and the reaction time is 12 minutes. The second micro-reaction mixer M2 and the second reaction module L2 are also placed at a preset temperature. After the Friedel-Crafts acylation reaction is completed, the obtained reaction solution (labeled as D reaction solution) is pumped into the second In the reaction mixer M2, the flow rate is 0.8 mL/min, and at the same time, the C solution is pumped into the second reaction mixer by the third advection pump P3 according to the flow rate ratio (2:1) of the above D reaction solution. In M2, the mixture is uniformly mixed and transferred to the second reaction module L2 (inner diameter: 3mm, length: 12.5m) for quenching reaction, the reaction temperature is 20° C., and the reaction time is 5 min. The reaction liquid obtained after quenching the reaction was pumped into the receiving reactor R, stirred for 5 min, flowed out through the liquid outlet O, collected in the liquid separation device, allowed to stand for liquid separation, the upper aqueous phase was extracted with dichloromethane, and the organic phases were combined , Adding an equal volume of saturated sodium carbonate solution for alkalization, extracting and separating the liquids, the resulting aqueous phase is acidified with concentrated hydrochloric acid to its pH value of 1. After extracting the aqueous phase with dichloromethane, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered with suction, concentrated under reduced pressure and dried under vacuum at 35°C for 12 hours to obtain a white solid 4-(4-methyl) Oxybenzoyl)butyric acid, the yield was 96%.
References:
CN112778117,2021,A Location in patent:Paragraph 0071-0076
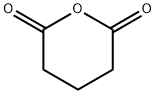
108-55-4
431 suppliers
$6.00/25g

13139-86-1
130 suppliers
$45.00/5g

4609-10-3
25 suppliers
$71.00/1g