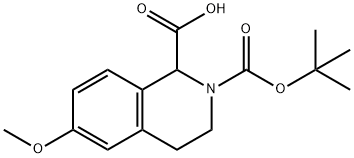
2-BOC-6-METHOXY-3,4-DIHYDRO-1H-ISOQUINOLINE-1-CARBOXYLIC ACID synthesis
- Product Name:2-BOC-6-METHOXY-3,4-DIHYDRO-1H-ISOQUINOLINE-1-CARBOXYLIC ACID
- CAS Number:499139-27-4
- Molecular formula:C16H21NO5
- Molecular Weight:307.34
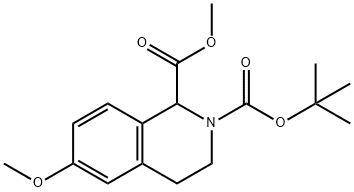
531556-16-8
8 suppliers
inquiry

499139-27-4
29 suppliers
$65.00/5mg
Yield:-
Reaction Conditions:
with methanol;lithium hydroxide monohydrate in tetrahydrofuran; for 12 h;
Steps:
Sodium hydride (0.098 g, 2.44 mmol) was added to 15A (0.500 g, 1.63 mmol) in DMF (5 mL) at 0 °C. The reaction was allowed to come to rt and stirred for 1 h before again cooling the mixture back to 0 °C. Iodomethane (0.10 mL, 1.63 mmol) was slowly added and the mixture allowed to raise to rt. After 16 h, the reaction mixture was diluted with EtOAc (50 mL) and treated with FLO (50 mL). The FLO layer was separated and extracted once again with EtOAc (50 mL). The combined organic extract was washed with brine, dried over Na2S04, filtered, and concentrated. Purification by silica gel chromatography gave colorless oil. The ester was hydrolyzed by dissolution in THF (10 mL), treatment with lithium hydroxide monohydrate (0.14 g, 3.25 mmol) (3 mL), and the addition of MeOH (1 mL). After 12 h, the mixture was diluted with FLO and organics concentrated. The remaining aqueous layer was made acidic with 10% citric acid and extracted with EtOAc (3 x 30 mL). The combined organic extract was washed with FLO, brine, dried over a2S04, filtered, and concentrated. This material and tert-butyl 4-aminobenzoate (0.314 g, 1.63 mmol) in pyridine (10 mL) was treated with POCI3 (0.15 mL, 1.63 mmol) at -10 °C. After stirring for 2 h, the reaction was quenched with H2O and extracted with EtOAc. The organic layer was washed with FLO, 1.0 M HCl solution, H20, brine, dried over Na2S04, filtered, and concentrated. The residue was treated with 50% TFA/DCM. After 2 h, the reaction was concentrated. Purification by reverse phase HPLC and concentrating in vacuo gave 16A as oil. MS (ESI) m/z: 327.3 (M+H)+.
References:
WO2013/55984,2013,A1 Location in patent:Paragraph 00287
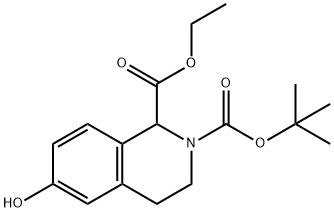
128073-49-4
17 suppliers
inquiry

499139-27-4
29 suppliers
$65.00/5mg
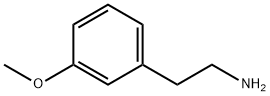
2039-67-0
243 suppliers
$17.46/5G

499139-27-4
29 suppliers
$65.00/5mg
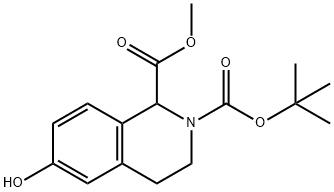
350014-19-6
10 suppliers
inquiry

499139-27-4
29 suppliers
$65.00/5mg
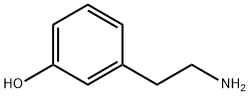
588-05-6
82 suppliers
$49.00/100mg

499139-27-4
29 suppliers
$65.00/5mg