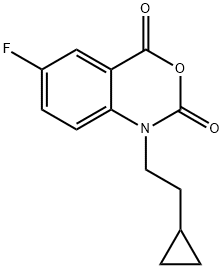
1-(2-CYCLOPROPYL-ETHYL)-6-FLUORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE synthesis
- Product Name:1-(2-CYCLOPROPYL-ETHYL)-6-FLUORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE
- CAS Number:477933-12-3
- Molecular formula:C13H12FNO3
- Molecular Weight:249.24
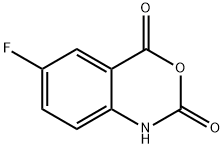
321-69-7
149 suppliers
$19.00/1g

2566-44-1
154 suppliers
$23.00/250mg
![1-(2-CYCLOPROPYL-ETHYL)-6-FLUORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE](/CAS/GIF/477933-12-3.gif)
477933-12-3
21 suppliers
$150.00/50mg
Yield:477933-12-3 84%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran;toluene at 20;Product distribution / selectivity;
Steps:
2; 20 1-(2-cyclopropylethyl)-6-fluorobenzo[d]-[1,3]oxazine-2,4-dione
Under nitrogen atmosphere, 6-fluoro-1H-benzo[d][1,3]oxazine-2,4-dione (2.2 g, 12.3 mmol), triphenylphosphine (3.5 g, 13.4 mmol) and cyclopropyl ethanol were stirred together in THF and treated with diethyl azodicarboxylate (40% in toluene, 6.14 ml, 13.4 mmol). The reaction was stirred overnight at room temperature and under nitrogen atmosphere. The THF was evaporated, and the crude compound was purified by silica gel chromatography (ethyl acetate/petroleum ether) to give intermediate 2 (2.54 g, 84%) which was a white powder. Intermediate 2 was characterized by the following spectroscopic data: 1H NMR (d6-DMSO, 400 MHz) δ (ppm) 0.34-0.41 (m, 3H), 0.65-0.89 (m,2H), 1.54 (q, J=7.28 Hz, 2H), 4.08 (t, J=7.28 Hz, 2H), 7.17 (td, J=8.54 Hz and J=2.14 Hz, 1H), 7.47 (dd, J=11.3 Hz and J=2.14 Hz, 1H), 8.07 (dd, J=8.54 Hz and J=6.22 Hz, 1H); and MS (ESI, EI-) m/z=222 (MH-) mass of acid derivate.; Under nitrogen atmosphere, intermediate 19 (12.3 mmol), triphenylphosphine (13.4 mmol) and cyclopropyl ethanol were stirred together in THF and treated with diethyl azodicarboxylate (40% in toluene, 13.4 mmol). The reaction was stirred overnight at room temperature and under nitrogen atmosphere. The THF was removed, and the crude compound was purified by silica gel chromatography to give intermediate 20, which was a white powder. Intermediate 20 was characterized by the following spectroscopic data: 1H NMR (DMSO-d6, 400 MHz) δ (ppm) 0.34-0.41 (m, 3H), 0.65-0.89 (m,2H), 1.54 (q, J=7.28 Hz, 2H), 4.08 (t, J=7.28 Hz, 2H), 7.17 (td, J=8.54 Hz and J=2.14 Hz, 1H), 7.47 (dd, J=11.3 Hz and J=2.14 Hz, 1H), 8.07 (dd, J=8.54 Hz and J=6.22 Hz, 1H); and MS (ESI, EI-) m/z=222 (MH-) mass of acid derivate.
References:
US2009/60866,2009,A1 Location in patent:Page/Page column 52; 56

446-08-2
388 suppliers
$14.00/5g
![1-(2-CYCLOPROPYL-ETHYL)-6-FLUORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE](/CAS/GIF/477933-12-3.gif)
477933-12-3
21 suppliers
$150.00/50mg
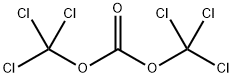
32315-10-9
418 suppliers
$10.00/1g
![Benzoic acid, 2-[(2-cyclopropylethyl)amino]-5-fluoro-](/CAS/20210305/GIF/477932-69-7.gif)
477932-69-7
0 suppliers
inquiry
![1-(2-CYCLOPROPYL-ETHYL)-6-FLUORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE](/CAS/GIF/477933-12-3.gif)
477933-12-3
21 suppliers
$150.00/50mg