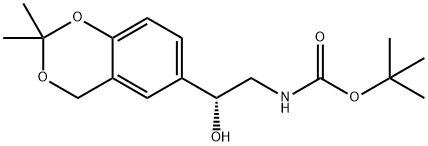
CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester synthesis
- Product Name:CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester
- CAS Number:452339-72-9
- Molecular formula:C17H25NO5
- Molecular Weight:323.38
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/CAS/20150408/GIF/452339-71-8.gif)
452339-71-8
65 suppliers
inquiry
![CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/452339-72-9.gif)
452339-72-9
65 suppliers
inquiry
Yield:452339-72-9 95.7%
Reaction Conditions:
with borane-THF;(R)-tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c]-[1,3,2]oxazaborole in tetrahydrofuran at -12 - -10; under 7500.75 Torr;Pressure;Temperature;
Steps:
1-3 Example 2:
(1) The concentrations of borane tetrahydrofuran and (R)-2-methyl-CBS-oxazolborane tetrahydrofuran are both1 mol/L, the tetrahydrofuran solution of ketone is configured to be 2 mol/L, wherein the mass ratio of ketone to tetrahydrofuran is 1:3.(2) Set the temperature value of the precooler in the microreactor device, the precooling temperature of the borane tetrahydrofuran solution is set to -10°C, and the (R)-2-methyl-CBS-oxazolborane tetrahydrofuran precooling temperature is set to -10°C, and the pre-cooling temperature of the reaction zone was set to -12°C.(3) Open the flow control valve of the tetrahydrofuran solution heat exchanger refrigerant inlet pipeline of the borane tetrahydrofuran heat exchanger in the precooling zone, (R)-2-methyl-CBS-oxazolborane tetrahydrofuran, and ketone, and start adjusting the equipment After the equipment temperature drops to the set value, turn on the raw material delivery pump in turn.(4) After the precooling of each district in the device is completed, turn on the borane tetrahydrofuran pump, pay attention to observe the change of the temperature in the reactor, and turn on (R)-2-methyl-CBS-oxazolborane when the temperature is stabilized at the set value The tetrahydrofuran pump, at this time, adjust the needle valve on the heat exchange sheet pipeline in the reactor device to control the temperature in the reaction sheet under the reaction conditions and maintain a stable state, wherein the reaction temperature of each microchannel reactor is -10 ° C, and the reaction time 8s, the reaction pressure is 1.0MPa.(5) control the flow of borane tetrahydrofuran by setting feeding pump to be 60mL/min,The flow rate of (R)-2-methyl-CBS-oxazolborane tetrahydrofuran was 6mL/min, and the molar ratio of (R)-2-methyl-CBS-oxazolborane and borane tetrahydrofuran was controlled to be 1:15, The flow rate of the ketone tetrahydrofuran solution is 25 mL/min, and the reacted mixture is passed through two microchannel reactors in turn to obtain a chiral reducing stream, and the obtained chiral reducing stream and the ketone tetrahydrofuran solution cooled to -10 ° C pass through two again. The microchannel reactor was reacted with a molar ratio of ketone in tetrahydrofuran and borane in tetrahydrofuran of 1:1.4.(6) The liquid chromatography test of the reaction solution showed that the conversion rate of the ketone solution in tetrahydrofuran was 100%.(7) get reaction solution 7kg, below 10 , drip methanol 750g quenching reaction, vacuum concentrating solvent, add 3kg ethyl acetate and 3kg water in residue, stir 1 hour at 10 , layer, organic phase is this Wash once with saturated brine and water, dry the organic phase, concentrate until no fractions flow out, add 1.5kg of petroleum ether, stir overnight, filter, and dry to obtain a white solid, yield 95.7%, chemical purity 99.93%, chiral purity 99.92% .
References:
CN113735816,2022,B Location in patent:Paragraph 0005; 0021-0054
![2-Bromo-1-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)ethanone](/CAS2/GIF/102293-80-1.gif)
102293-80-1
83 suppliers
$265.00/250mg
![CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/452339-72-9.gif)
452339-72-9
65 suppliers
inquiry
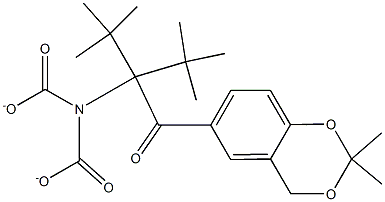
452339-70-7
9 suppliers
inquiry
![CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/452339-72-9.gif)
452339-72-9
65 suppliers
inquiry
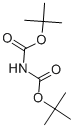
51779-32-9
283 suppliers
$5.00/1g
![CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/452339-72-9.gif)
452339-72-9
65 suppliers
inquiry
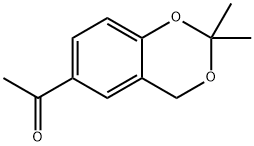
54030-34-1
5 suppliers
inquiry
![CarbaMic acid, [(2R)-2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/452339-72-9.gif)
452339-72-9
65 suppliers
inquiry