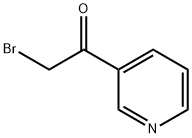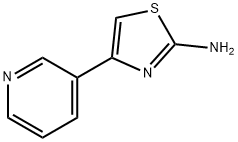
4-PYRIDIN-3-YL-THIAZOL-2-YLAMINE synthesis
- Product Name:4-PYRIDIN-3-YL-THIAZOL-2-YLAMINE
- CAS Number:30235-27-9
- Molecular formula:C8H7N3S
- Molecular Weight:177.23
Yield: 92%
Reaction Conditions:
Stage #1:methyl-3-pyridylketone with hydrogen bromide;acetic acid at 0; for 0.25 h;Inert atmosphere;
Stage #2: with bromine at 70; for 1 h;Inert atmosphere;
Stage #3:thiourea in ethanolInert atmosphere;Reflux;
Steps:
4.2.5 General procedure for the preparation of compound 8
General procedure: tep 1. To a 0°C solution of 4-acetylpyridine or 3-acetylpyridine (4.90g, 41.30mmol) and 48% HBr (7.0mL) in acetic acid (46.0mL) was added, dropwise over 15min, a solution of Br2 (2.30mL, 45.00mmol) in acetic acid (8.0mL). After the addition was complete, the mixture was allowed to warm to r.t. and then was heated at 70°C for 1h. The mixture was cooled to 0°C and treated with diethyl ether. The resultant white solid was isolated by vacuum filtration to give the bromoketone as the HBr salt, which was used in next step as obtained.Step 2. A mixture of above salt (10.00g, 35.59mmol) and thiourea (2.71g, 35.50mmol) in absolute EtOH (100mL) was refluxed overnight. After cooling, the reaction mixture was diluted with water (400mL), the pH was adjusted to 11 with ammonium hydroxide solution, and was further stirred for 2h. The resulting precipitate was filtered, washed, and dried to provide title compound 8 as a yellow solid (about 90% yield).
References:
Fan, Meixia;Ma, Shuchao;Ouyang, Ben;Qi, Junhui;Wang, Linan;Yao, Lei [Bioorganic and medicinal chemistry,2020,vol. 28,# 19]
2510-23-8
172 suppliers
$20.00/1g
17356-08-0
3 suppliers
inquiry

30235-27-9
85 suppliers
$45.00/50mg
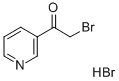
17694-68-7
199 suppliers
$10.00/1g

17356-08-0
3 suppliers
inquiry

30235-27-9
85 suppliers
$45.00/50mg

350-03-8
651 suppliers
$10.00/5g
144-55-8
1312 suppliers
$10.00/25g

30235-27-9
85 suppliers
$45.00/50mg