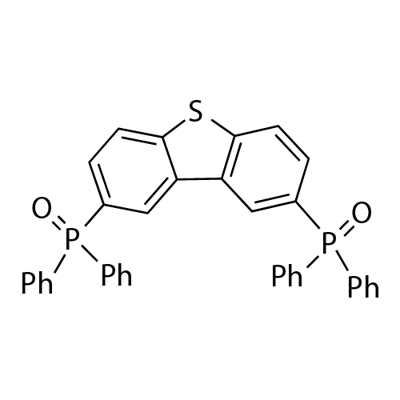
4'-PHOSPHOPANTETHEINE synthesis
- Product Name:4'-PHOSPHOPANTETHEINE
- CAS Number:1019842-99-9
- Molecular formula:C36H26O2P2S
- Molecular Weight:584.6
31574-87-5
214 suppliers
$7.00/250mg
1079-66-9
421 suppliers
$5.00/5g

1019842-99-9
94 suppliers
$40.00/100mg
Yield:1019842-99-9 72%
Reaction Conditions:
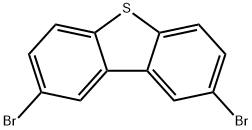
Stage #1: 2,8-dibromodibenzothiophenewith n-butyllithium in tetrahydrofuran at -80; for 2 h;Inert atmosphere;
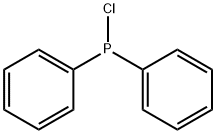
Stage #2: chloro-diphenylphosphine in tetrahydrofuran at 20; for 12 h;Inert atmosphere;
Stage #3: with dihydrogen peroxide in dichloromethane;water at 20; for 2 h;Inert atmosphere;
Steps:
2
5 g of 2,8-dibromodibenzothiophene, 100 mL of ultra-dry tetrahydrofuran, under anhydrous, oxygen-free, nitrogen protection conditions, cooling when the temperature reaches -80°C , the temperature is constant and under the condition of -80°C , under the protection of nitrogen, slowly drip 2.5 M tert-butyllithium 35.04 mmol, keep it at -80°C for two hours after lithiation, slowly add 6.44 g of diphenylphosphorus chloride, and then slowly return to room temperature. The system was stirred for 12 hours at room temperature under nitrogen protection. After the reaction, 500 mL of methanol was added to the system and 1000 mL of dichloromethane was added to the system. The organic phase was extracted with methane and 1000 mL of water. The organic phase was separated and concentrated to 5 mL. The system was then dissolved in 30 mL of dichloromethane. Under stirring at room temperature, 10 mL of 30% hydrogen peroxide aqueous solution was slowly added dropwise. A large amount of white was precipitated in the system. Solid, after reacting for 2 hours, the organic solvent in the system was removed by rotary evaporation to obtain a white crude product, which was separated by column chromatography with dichloromethane and methanol (volume ratio 20:1) eluent to obtain the target product, and then sublimated by vacuum 6.14 g of white product (II)-1 was obtained (72% yield).
References:
CN113461545,2021,A Location in patent:Paragraph 0046-0049
1258319-81-1
1 suppliers
inquiry

1019842-99-9
94 suppliers
$40.00/100mg

132-65-0
378 suppliers
$6.00/5g

1019842-99-9
94 suppliers
$40.00/100mg
