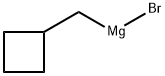
4-penten-1-ylmagnesium bromide synthesis
- Product Name:4-penten-1-ylmagnesium bromide
- CAS Number:34164-50-6
- Molecular formula:C5H9BrMg
- Molecular Weight:173.35
Yield:-
Reaction Conditions:
with iodine in tetrahydrofuran at 45 - 50; for 0.25 h;Inert atmosphere;
Steps:
B1.B2.1 Preparation of Intermediate mixture B1 and B2 Steps 1 and 2
Preparation of Intermediate mixture B1 and B2. B1 B2 Steps 1 and 2: Preparation of racemate B1-1 : Magnesium metal (1 .32 g, 54.3 mmol) was added to a 2-neck flask fitted with a reflux condenser and the vessel was flushed with Ar. THF (42 mL) was added followed by iodine (ca. 5 mg). The stirred suspension was heated to 45 °C and 5-bromopent-1 -ene was added (1 .2 g, 8.1 mmol) in one portion. After stirring several minutes, additional 5-bromopent-1 -ene (5.5 g, 37 mmol) was added at a rate sufficient to maintain gentle reflux. The resulting mixture was stirred at 50 °C for 15 min and was then cooled to ambient temperature and used immediately in the following step. A suspension of Cul (630 mg, 3.3 mmol) in THF (24 mL) under Ar was cooled to - 5 °C. An aliquot of pent-4-enylmagnesium bromide (ca. 0.95 M, 20 mL, 19 mmol) prepared in step 1 was added over 5 min, and the resulting mixture was stirred for an additional 15 min. The reaction mixture was then cooled to -20 °C, and (±)-exo-2,3-epoxynorbornane (1 .5 g, 14 mmol) was added as a solution in THF (5 mL) over 1 min. Two additional portions of THF (2.5 mL each) were used to ensure complete transfer, and the resulting mixture was stirred for 20 min. The reaction was then removed from the cold bath and warmed to rt. After stirring an additional 1 .75 h, the reaction was quenched with saturated aqueous NH CI (5 mL) and was filtered with EtOAc (100 mL) and H2O (100 mL) through Celite. The phases were separated, and the organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford (±)-B1 -1.. 1 H-NMR (300 MHz, CDCIs) δ 5.90 - 5.67 (m, 1 H), 5.04 - 4.86 (m, 2H), 3.12 (s, 1 H), 2.20 - 1 .92 (m, 5H), 1 .69 - 1 .57 (m, 1 H), 1 .55 - 1 .12 (m, 9H), 1 .03 - 0.84 (m, 1 H).
References:
GILEAD SCIENCES, INC.;BJORNSON, Kyla;KARKI, Kapil K.;LINK, John O.;PYUN, Hyung-Jung;SCHRIER, Adam J.;STEVENS, Kirk L.;TAYLOR, James G.;VIVIAN, Randall W.;ZABLOCKI, Jeff;ZIPFEL, Sheila WO2014/145095, 2014, A1 Location in patent:Page/Page column 94
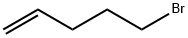
1119-51-3
369 suppliers
$12.00/5g

34164-50-6
11 suppliers
inquiry
123245-94-3
3 suppliers
inquiry

34164-50-6
11 suppliers
inquiry