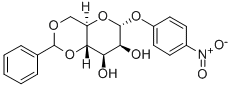
4-Nitrophenyl4,6-O-benzylidene-a-D-mannopyranoside synthesis
- Product Name:4-Nitrophenyl4,6-O-benzylidene-a-D-mannopyranoside
- CAS Number:58056-41-0
- Molecular formula:C19H19NO8
- Molecular Weight:389.36
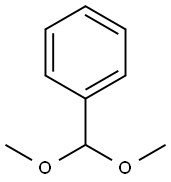
1125-88-8
353 suppliers
$6.00/25g
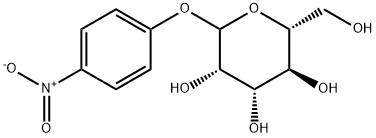
10357-27-4
159 suppliers
$32.00/500mg

58056-41-0
18 suppliers
inquiry
Yield:58056-41-0 83%
Reaction Conditions:
with triethylamine in N-methyl-acetamide;diethyl ether;
Steps:
1.41.a 1.41.a
1.41.a p-Nitrophenyl 4,6-O-benzylidene-α-D-mannopyranoside Benzaldehyde dimethyl acetal (3.2 ml, 21.4 mmol) and a 54% strength solution of tetrafluoboric acid in diethyl ether (2.7 ml, 20 mmol) are added to a solution of p-nitrophenyl α-D-mannopyranoside (6.0 g, 20 mmol) in dimethylformamide (120 ml). The mixture is stirred at room temperature for 5 hours, the reaction is then interrupted by addition of triethylamine (2.8 ml, 20 mmol) and the mixture is concentrated in vacuo. After flash chromatography [toluene→toluene/ethanol 20:1], colourless crystals (6.48 g, 83%) are obtained; TLC [methylene chloride/methanol/ammonia (25%) 15:3:0.2]: Rf=0.82; [α]20=+170.7° (c=1.0/CH2Cl2); melting point=116° C.
References:
US6271342,2001,B1

100-52-7
950 suppliers
$21.30/2g

10357-27-4
159 suppliers
$32.00/500mg

58056-41-0
18 suppliers
inquiry