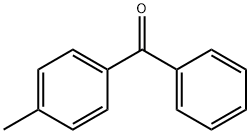
4-Methylbenzophenone synthesis
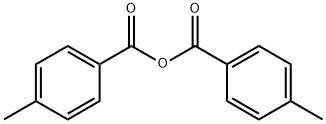
- Product Name:4-Methylbenzophenone
- CAS Number:134-84-9
- Molecular formula:C14H12O
- Molecular Weight:196.24
Yield:134-84-9 93%
Reaction Conditions:
with [Pd(2-((1-naphthalenyl)methylene)-N-phenylhydrazinecarbothioamide)Cl(PPh3)];caesium carbonate in toluene at 110; for 12 h;
Steps:
General Procedure for Coupling of Arylboronic Acids with Aromatic Aldehydes
General procedure: Arylboronic acid (1 mmol), aromatic aldehyde (1.5 mmol), Cs2CO3 (3.0 mmol), catalyst (5.0 mol%) and toluene (3.0 ml) were taken in a round-bottom flask. The mixture was then heated under reflux for 12 h under aerobic conditions and monitored by TLC. At the end of the specified time, the reaction mixture was cooled to room temperature, quenched with 1 N HCl (5 ml) and finally extracted with ethyl acetate (2 × 5 ml). The combined extracts were dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The residue obtained was subjected to flash chromatography on a silica gel column to purify the desired ketone.
References:
Prabhu, Rupesh Narayana;Ramesh, Rengan [Tetrahedron Letters,2017,vol. 58,# 5,p. 405 - 409] Location in patent:supporting information
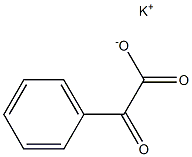
201230-82-2
1 suppliers
inquiry
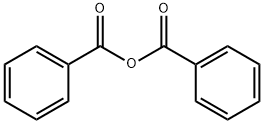
106-38-7
405 suppliers
$14.00/5g

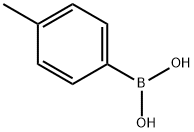
98-80-6
738 suppliers
$5.00/5g

134-84-9
474 suppliers
$7.00/10g