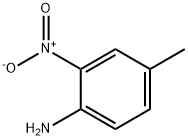
4-Methyl-2-nitroaniline synthesis
- Product Name:4-Methyl-2-nitroaniline
- CAS Number:89-62-3
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
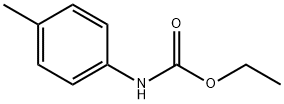
5255-66-3
8 suppliers
inquiry

89-62-3
226 suppliers
$13.00/25g
Yield: 80%
Reaction Conditions:
with tert.-butylnitrite;palladium diacetate in 1,4-dioxane at 90;Reagent/catalyst;
Steps:
1 Example 1
4-methylaniline was amino-protected with ethyl chloroformate to produce N-(p-toluene) ethyl carbamate; then in a 35.0 mL pressure tube,Ethyl N-(p-toluene)carbamate 0.10 mmol, palladium acetate 0.01 mmol, t-butyl nitrite 0.60 mmol, 1,4-dioxane 2 mL,With air as an oxidant, placed in an oil bath, the reaction was heated to 90°C;The reaction was monitored by TLC (ethyl acetate: V, petroleum ether = 1:10); the organic phase was extracted with ethyl acetate after 6 h of reaction, the organic phases were combined, washed with water (3×10 mL), dried over anhydrous sodium sulfate, and filtered by suction. Distillation under reduced pressure to give a crude product, using ethyl acetate: petroleum ether = 1:20 mixed organic solvent as eluent, and silica gel column purification, isolated yield of 80%;Finally, the target product 4-methyl-2-nitroaniline is obtained by hydrolysis under acidic conditions.
References:
Nanjing University of Science and Technology;Nanjing University Of Science And Technology Lianyungang Institute;Peng Xinhua;Gao Xi;Pan Su;Liu Shunqiang;Jiang Zhibin CN107915646, 2018, A Location in patent:Paragraph 0020-0023; 0024-0027; 0028-0031; 0032-0035
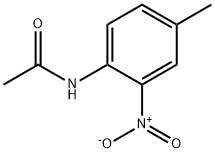
612-45-3
26 suppliers
$32.29/1g

89-62-3
226 suppliers
$13.00/25g
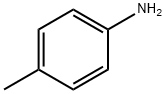
106-49-0
396 suppliers
$5.00/5 g

89-62-3
226 suppliers
$13.00/25g
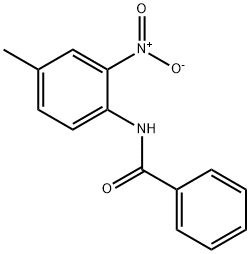
26242-93-3
0 suppliers
inquiry

89-62-3
226 suppliers
$13.00/25g
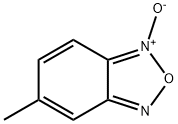
19164-41-1
30 suppliers
$15.00/1g
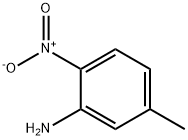
578-46-1
132 suppliers
$6.00/250mg

89-62-3
226 suppliers
$13.00/25g