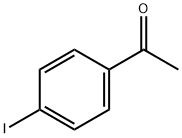
4'-Iodoacetophenone synthesis
- Product Name:4'-Iodoacetophenone
- CAS Number:13329-40-3
- Molecular formula:C8H7IO
- Molecular Weight:246.05

149104-90-5
297 suppliers
$10.00/250mg

13329-40-3
273 suppliers
$6.00/5g
Yield:13329-40-3 91%
Reaction Conditions:
with iodine;potassium carbonate in acetonitrile at 80; for 8 h;Inert atmosphere;Schlenk technique;Sealed tube;
Steps:
General procedure: Arylboronic acid 1 (0.5 mmol) and K2CO3 (1 mmol, 138.0mg) were added to a 20 mL Schlenk-tube equipped with amagnetic stir bar. The tube was evacuated twice and backfilledwith N2. MeCN (2 mL) and I2 (0.75 mmol, 191 mg)were added to the tube at r.t. under a stream of N2, and thetube was sealed and placed into a pre-heated oil bath at 80 °Cfor 8-12 h. The resulting solution was cooled to r.t. and H2O(10 mL) was added. The aq layer was extracted with EtOAc (3 × 5 mL). For products 2s and 2t, HCl (1 M) was added tothe aq solution until pH 2 before extraction. The combinedorganic phase was dried over anhydrous Na2SO4, filteredand concentrated by rotary evaporation. Purification of theresidue by column chromatography on silica gel providedthe desired product 2a-v
References:
Niu, Liting;Zhang, Hao;Yang, Haijun;Fu, Hua [Synlett,2014,vol. 25,# 7,art. no. 691,p. 995 - 1000] Location in patent:supporting information

99-90-1
477 suppliers
$6.00/25g

13329-40-3
273 suppliers
$6.00/5g

99-92-3
522 suppliers
$6.00/10g

13329-40-3
273 suppliers
$6.00/5g
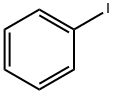
591-50-4
491 suppliers
$10.00/1g
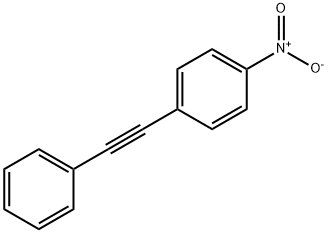
1942-30-9
21 suppliers
inquiry
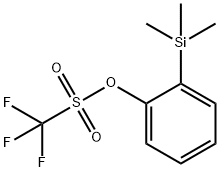
88284-48-4
171 suppliers
$9.00/250mg

13329-40-3
273 suppliers
$6.00/5g

591-50-4
491 suppliers
$10.00/1g

75-36-5
583 suppliers
$17.92/100G

13329-40-3
273 suppliers
$6.00/5g