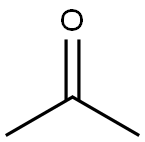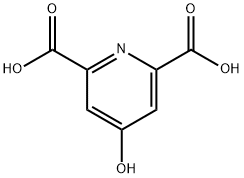
4-Hydroxypyridine-2,6-dicarboxylic acid synthesis
- Product Name:4-Hydroxypyridine-2,6-dicarboxylic acid
- CAS Number:499-51-4
- Molecular formula:C7H5NO5
- Molecular Weight:183.12
Yield: 92.3%
Reaction Conditions:
Stage #1:Dimethyl oxalate;acetone with sodium ethanolate in ethanol at 70; for 2 h;
Stage #2: with hydrogenchloride in water at 100;
Stage #3: with ammonium hydroxide at 0; for 48 h;Reagent/catalyst;Temperature;
Steps:
1-3; 1-3 A method for preparing 4-hydroxypyridine-2,6-dicarboxylic acid, comprising the steps of:
a, in a three-necked bottle A with a reflux condenser and a drying tube,102 g of a 20% sodium ethoxide ethanol solution was added and stirred well.b. Pre-add 8.54 g of acetone and 36.6 g of dimethyl oxalate in a three-necked bottle B, and add ethanol dropwise at room temperature until the dimethyl oxalate is completely dissolved, and it is reserved for use.c. Premix the acetone in the three-neck bottle B.A mixed droplet of dimethyl oxalate and ethanol is added to a three-neck bottle A solution of sodium ethoxide in ethanol,The reaction mixture gradually turns yellow during the addition.When the mixture added to the three-neck bottle B still has 25% remaining,Heat to 70 ° C. After the end of the addition, continue stirring for 2 hours.A yellow viscous paste is obtained. The system was cooled to 0 to 5 ° C, 52 mL of concentrated hydrochloric acid and 40 g of crushed ice were added to adjust pH = 7, and filtered.Filter out salts that are not completely dissolved,Stir at room temperature overnight,Excess ethanol was distilled off under reduced pressure.d, adding 60 mL of concentrated hydrochloric acid to the mixture system prepared in the above step, heating to 100 ° C, reacting for 5 to 10 hours, cooling to 5 ° C, dropping 155 mL of ice water, stirring and filtering, washing with a small amount of ice water, heating with solid water The activated carbon was decolorized, filtered while hot, recrystallized by gradient cooling, and white needle-like crystals were filtered and vacuum dried for use.e, 21.1g of the white needle crystal prepared in the step is added to a 250ml three-necked bottle C, the system is cooled to 0 ° C, and 145mL of ammonia water is added dropwise. After the dropwise addition, the suspension is heated to room temperature, stirred for 48 hours, and excess is removed under reduced pressure. Ammonia.f. Add 60 mL of water to the three-necked bottle C, add decolorization by heating with activated carbon, filter while hot, adjust the temperature of the filtrate to pH=1 with concentrated hydrochloric acid, obtain a white precipitate, cool the system to 0-5 ° C, and wash with a small amount of ice water. The solid was further added with 96 ml of water, boiled for 30 min, naturally cooled to room temperature, filtered to give white crystals, dried in vacuo.The yield was 92.3%.
References:
Jiutai Energies (Zhungeer) Co., Ltd.;Li Qi;Zhang Xiaolong;Li Quanfa;Zhang Qirong;Bian Xiuying;Li Xianliang CN110204482, 2019, A Location in patent:Paragraph 0028-0062
99-32-1
193 suppliers
$9.00/250mg

499-51-4
74 suppliers
$9.00/1g
68854-18-2
16 suppliers
$189.00/250mg

499-51-4
74 suppliers
$9.00/1g

4722-94-5
113 suppliers
$6.00/250mg

499-51-4
74 suppliers
$9.00/1g

2683-49-0
49 suppliers
$179.00/100mg

499-51-4
74 suppliers
$9.00/1g