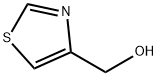
4-Hydroxymethylthiazole synthesis
- Product Name:4-Hydroxymethylthiazole
- CAS Number:7036-04-6
- Molecular formula:C4H5NOS
- Molecular Weight:115.15
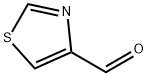
3364-80-5
195 suppliers
$11.00/250mg

7036-04-6
134 suppliers
$26.00/250mg
Yield: 74.3%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0; for 1 h;
Steps:
40.1 Thiazol-4-ylmethanol
To a solution of thiazole-4-carboxaldehyde (0.810 ml, 9.67 mmol, Combi-Blocks Inc.) in MeOH (48.3 ml) at 0 °C was added sodium borohydride (0.341 ml, 9.67 mmol, Sigma-Aldrich Chemical Company, Inc.) in portions. The reaction mixture was allowed to stir for 1 hour. Saturated aqueous ammonium chloride solution was carefully added and the reaction mixture was filtered. The filtrate was concentrated in vacuo. The solid was taken up in 10% MeOH/DCM and filtered through a plug of silica gel to provide thiazol- 4-ylmethanol (0.826 g, 7.18 mmol, 74.3% yield) as a yellow oil. MS m/z = 116.0 [M+H]+. Calculated for C4H5NOS: 115.009. H NMR (400 MHz, CHLOROFORM -J) δ ppm 2.58 (br. s., 1 H) 4.86 (s, 2 H) 7.27 - 7.30 (m, 1 H) 8.83 (d, J=l .76 Hz, 1 H)
References:
AMGEN INC.;MINATTI, Ana Elena;LOW, Jonathan, D.;ALLEN, Jennifer, R.;AMEGADZIE, Albert;BROWN, James;FROHN, Michael, J.;GUZMAN-PEREZ, Angel;HARRINGTON, Paul, E.;LOPEZ, Patricia;MA, Vu Van;NISHIMURA, Nobuko;QIAN, Wenyuan;RUMFELT, Shannon;RZASA, Robert, M.;SHAM, Kelvin;SMITH, Adrian, L.;WHITE, Ryan;XUE, Qiufen WO2014/138484, 2014, A1 Location in patent:Page/Page column 167; 168
14527-43-6
183 suppliers
$9.00/1g

7036-04-6
134 suppliers
$26.00/250mg
3973-08-8
257 suppliers
$6.00/1g

7036-04-6
134 suppliers
$26.00/250mg
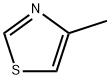
693-95-8
337 suppliers
$6.00/5g

7036-04-6
134 suppliers
$26.00/250mg
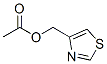
90724-59-7
1 suppliers
inquiry

7036-04-6
134 suppliers
$26.00/250mg