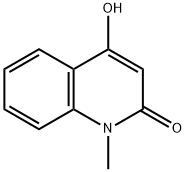
4-HYDROXY-1-METHYL-2-QUINOLONE synthesis
- Product Name:4-HYDROXY-1-METHYL-2-QUINOLONE
- CAS Number:1677-46-9
- Molecular formula:C10H9NO2
- Molecular Weight:175.18
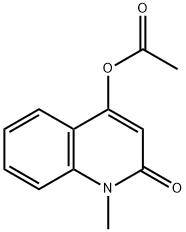
54350-10-6
0 suppliers
inquiry

1677-46-9
111 suppliers
$14.00/1g
Yield: 100%
Reaction Conditions:
with sodium hydroxide in ethanol
Steps:
18 Synthesis of Derivative 12 of Hirtellanine Beta
N-methylaminobenzoic acid (500 mg, 3.3 lmmo 1)Was dissolved in 8 ml of acetic anhydride and acetic acid (1: 1, v / v)Of the mixed solution,The reaction solution was refluxed for 3 hours.The reaction was quenched by the slow addition of 10 ml of ice water and extracted three times with 15 ml each of ethyl acetate. The combined organic layers were washed once with 20 ml of saturated brine and the solvent was evaporated under reduced pressure. The resulting solid was dissolved in 20 ml of ethanol (0.1 eq) and stirred for 10 minutes. Ethanol was evaporated to dryness under reduced pressure and purified by column chromatography to give the intermediate 4-Hydroxy-l-methyl-lH-quinolin-2-one, which was further treated in the same manner as in Example 9 Method A The title compound derivative 12 (597 mg, yield 64%) was obtained.
References:
Basilea Pharmaceutica China Ltd;SHEN, ZHENGWU;ZHENG, SHUYAN;ZHANG, JINGHUA;SHOU, QINGYAO CN103319497, 2016, B Location in patent:Paragraph 0059; 0079
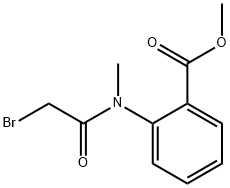
5946-42-9
3 suppliers
inquiry

1677-46-9
111 suppliers
$14.00/1g
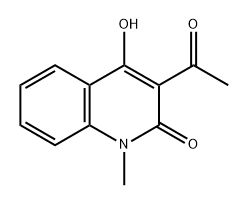
54289-76-8
13 suppliers
inquiry

1677-46-9
111 suppliers
$14.00/1g
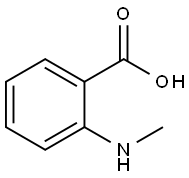
119-68-6
124 suppliers
$25.30/25g
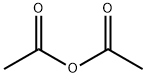
108-24-7
5 suppliers
$14.00/250ML

1677-46-9
111 suppliers
$14.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1677-46-9
111 suppliers
$14.00/1g
