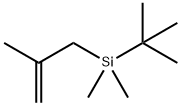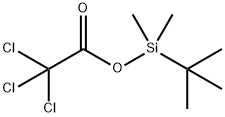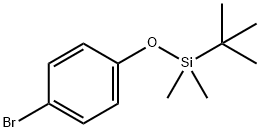
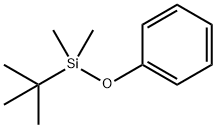
(4-BROMOPHENOXY)-TERT-BUTYLDIMETHYLSILANE synthesis
- Product Name:(4-BROMOPHENOXY)-TERT-BUTYLDIMETHYLSILANE
- CAS Number:67963-68-2
- Molecular formula:C12H19BrOSi
- Molecular Weight:287.27

106-44-5
476 suppliers
$14.00/5g
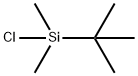
18162-48-6
669 suppliers
$9.00/5g

67963-68-2
132 suppliers
$17.00/1g
Yield: 56%
Reaction Conditions:
Stage #1:p-cresol;tert-butyldimethylsilyl chloride with 1H-imidazole in DMF (N,N-dimethyl-formamide) at 40; for 2 h;
Stage #2: with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane for 0.5 h;Sun lamp;Heating / reflux;
Steps:
Phenolic stilbene 57Z was obtained via a route employing a TBS-protecting group (Scheme 3). TBS-protected benzylic bromide 58 (Giral, L.; Montginoul, C.; Schue, R. S. et F. Bull. Soc. Chim. Belg. 1990, 99, 147-166) was prepared in 2 steps by TBS protection (Stern, A. J.; Swenton, J. S. J. Org. Chem. 1989, 54, 2953-2958) and NBS bromination. Phosphonium bromide 59 (Giral, L.; Montginoul, C.; Schue, R. S. et F. Bull. Soc. Chim. Belg. 1990, 99, 147-166) was prepared by two different methods: In one method, a neat mixture of benzylic bromide 58 and PPh3 was heated to melt with a heat gun for a few minutes to complete the reaction. (Stern, A. J.; Swenton, J. S. J. Org. Chem. 1989, 54, 2953-2958.) The second method was heating up the reaction mixture of benzylic bromide 58 and PPh3 at 8090° C. in THF over 6 hours. [00054] The Wittig reaction of 4-biphenylcarboxaldehyde with the phosphonium ylide generated from the phosphonium bromide 59 by the deprotonation using KHMDS provided the TBS protected stilbene 60Z in 44% yield. Desilylation with tetra-n-butylammonium fluoride (TBAF) (Corey, E. J.; Venkateswarlu, A. J. Am. Chem. Soc. 1972, 94, 6190-6191) in THF afforded phenol 57Z in quantitative yield. Rf0.24 (Hexanes/CH2Cl2 1/1); mp 97-103° C.; IR (KBr) 3530, 1608, 1508, 1485, 1255, 1171 cm-1; 1H NMR (300 MHz, CDCl3) δ7.61 (d, 2 H, J=7.3 Hz), 7.51-7.30 (m, 7 H), 7.21 (d, 2 H, J=8.5 Hz), 6.72 (d, 2 H, J=8.5 Hz), 6.55 (AB q, 2 H, J=12.6 Hz); 13C NMR (75 MHz, CDCl3) δ154.55, 140.64, 139.52, 136.46, 130.35, 129.81, 129.24, 128.74, 128.41, 127.23, 126.84, 126.81, 115.11; HRMS calcd for C20H16O 272.1201, found 272.1201.
References:
University of Pittsburgh US6706839, 2004, B1 Location in patent:Page column 15
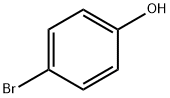
106-41-2
497 suppliers
$13.00/5g

18162-48-6
669 suppliers
$9.00/5g

67963-68-2
132 suppliers
$17.00/1g
18052-27-2
1 suppliers
inquiry

67963-68-2
132 suppliers
$17.00/1g