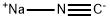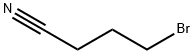
4-BROMOBUTYRONITRILE synthesis
- Product Name:4-BROMOBUTYRONITRILE
- CAS Number:5332-06-9
- Molecular formula:C4H6BrN
- Molecular Weight:148
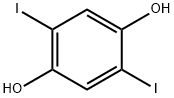
13064-64-7
74 suppliers
$22.00/250mg

5332-06-9
144 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in N,N-dimethyl-formamide
Steps:
Synthesis of Diiododialkyoxynitrile Benzene (3) (See FIG. 3)
9917-61 (R=-(CH2)3CN), 9917-89 (R=-(CH2)5CN), 9873-11 (R=-(CH2)7CH3)
Synthesis of Diiododialkyoxynitrile Benzene (3) (See FIG. 3)
To a 500 ml schlenk flask under argon fitted with a stir bar was added 2 (1 eq.), the selected bromoalkylnitrile or bromoalkane (2.02 eq.), and anhydrous DMF (0.3 M).
This mixture was deoxygenated via the freeze-pump-thaw method (3 times) then backfilled with argon.
Freshly ground sodium hydroxide (6.63 g, 166 mmol) was added and the reaction was stirred at room temperature for 18 hr or until complete as indicated by TLC (SiO2: Rf=0.4, 30% ethyl acetate/hexanes).
The crude reaction mixture was precipitated into 1M HCl, filtered via suction filtration, and dried under vacuum to yield 3 as a light tan powder which was used without further purification.
3a, whereby R=-(CH2)3CN:
The reagents used: 2 (10.00 g, 27.6 mmol) and 4-bromobutyronitrile (8.26 g, 55.8 mmol) yielded 3a (12.48 g, 91%).
1H NMR (300 MHz, CDCl3): δ 7.17 (s, 2H), 4.04 (t, 4H, J=5.58 Hz), 2.68 (t, 4H, J=7.10 Hz), 2.15 (q, 4H, J=5.82 Hz).
13C NMR (60 MHz, CDCl3): δ 152.44, 122.87, 119.01, 86.23, 67.47, 25.43, 14.33.
References:
Ricks-Laskoski, Holly L.;Snow, Arthur W;Laskoski, Matthew;Deese, Stephen M.;Chaloux, Brian L. US2014/39132, 2014, A1 Location in patent:Page/Page column

38694-47-2
17 suppliers
inquiry

5332-06-9
144 suppliers
$10.00/1g

628-20-6
247 suppliers
$10.00/10g

5332-06-9
144 suppliers
$10.00/1g