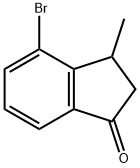
4-bromo-3-methyl-2,3-dihydro-1H-inden-1-one synthesis
- Product Name:4-bromo-3-methyl-2,3-dihydro-1H-inden-1-one
- CAS Number:102539-53-7
- Molecular formula:C10H9BrO
- Molecular Weight:225.08
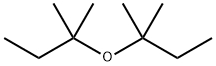
67000-01-5
1 suppliers
inquiry
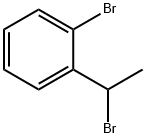
62384-31-0
17 suppliers
inquiry

105-53-3
743 suppliers
$5.00/25g

102539-53-7
16 suppliers
inquiry
Yield:102539-53-7 101 g (86%)
Reaction Conditions:
with potassium hydroxide;thionyl chloride;sodium ethanolate;AlCl3 in ethanol;dichloromethane;water;
Steps:
20 4-Bromo-3-methylindan-1-one
4-Bromo-3-methylindan-1-one To a solution of sodium ethoxide in ethanol obtained from 15.4 g (0.67 mmol) of sodium and 360 ml of anhydrous ethanol, a solution of 215 g (1.34 mmol) of diethyl malonate in 240 ml of ethanol was added dropwise, while vigorously stirring, over 15 min. Then, 137 g (0.52 mmol) of 1-bromo-2-(1-bromoethyl)benzene in 50 ml of ethanol was added dropwise with such a rate, so the reaction mixture would be slowly refluxing. The resulting mixture was additionally refluxed for 4 h, then cooled to room temperature, and a solution of 105 g of potassium hydroxide in 280 ml of water was added. This mixture was refluxed for 3 h, and then ethanol was distilled off at atmospheric pressure. The solution obtained was cooled to ambient temperature and acidified by saturated hydrochloric acid to pH 1. The precipitate formed was filtered off, washed with 2*200 ml of cold water, and dried in air. The dibasic acid obtained was then decarboxylated by heating it at 160° C. for 2 h. To the viscous oil obtained, 130 ml of SOCl2 was added, and the resulting mixture was stirred for 24 h at room temperature. The excess of SOCl2 was distilled off, and the residue was dissolved in 160 ml of anhydrous dichloromethane. The solution obtained was added dropwise to a suspension of 80.0 g (0.60 mmol) of AlCl3 in 800 ml of dichloromethane for 1 h at 0° C. The reaction mixture was refluxed for 3 h, then cooled to room temperature, poured on 500 cm3 of ice, and finally acidified by saturated HCl to pH 3. The organic layer was separated, and the aqueous layer was washed with 3*300 ml of methyl-tert-butyl ether. The combined organic extract was dried over K2CO3 and then evaporated to dryness. The title product was isolated fractional distillation in vacuum, bp 108-112° C./3 mm Hg. Yield 101 g (86%). Anal. calc. for C10H9BrO: C, 53.36; H, 4.03. Found: C, 53.48; H, 3.90. 1H NMR (CDCl3): δ 7.75 (dd, J=7.8 Hz, J=0.6 Hz, 1H, 7-H), 7.68 (dd, J=7.5 Hz, J=0.6 Hz, 1H, 5-H), 7.26 (t, J=7.6 Hz, 1H, 6-H), 3.48-3.58 (m, 1H, 3-H), 2.96 (dd, J=19.0 Hz, J=7.8 Hz, 1H, 2-H), 2.39 (dd, J=19.0 Hz, J=1.6 Hz, 1H, 2'-H), 2.43 (d, J=7.1 Hz, 3H, 2-CH3). 13C NMR (CDCl3): δ 205.4, 158.3, 138.3, 129.3, 122.6, 121.4, 109.6, 45.6, 34.0, 20.6.
References:
US2007/135595,2007,A1
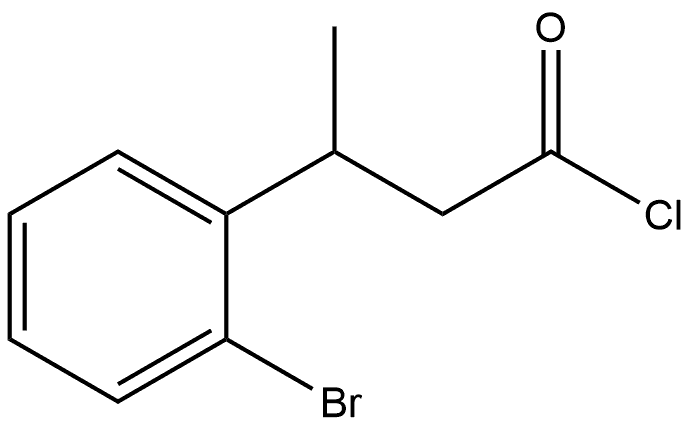
99057-75-7
0 suppliers
inquiry

102539-53-7
16 suppliers
inquiry