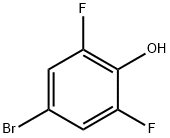
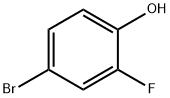
4-Bromo-2,6-difluorophenol synthesis
- Product Name:4-Bromo-2,6-difluorophenol
- CAS Number:104197-13-9
- Molecular formula:C6H3BrF2O
- Molecular Weight:208.99
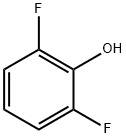
28177-48-2
306 suppliers
$15.00/5g

104197-13-9
245 suppliers
$6.00/1g
Yield:104197-13-9 93%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0 - 20; for 20 h;
Steps:
110
Intermediate 110 4-bromo-2,6-difluorophenol To a solution of 2,6-Difluorophenol (10.0 g, 76.86 mmoles) in DMF (60 mll), N-bromosuccinimide (13.68 g, 76.86 mmoles) was added at 0° C. and stirred at RT for 20 h. The reaction mixture was concentrated, diluted with water and extracted with ethyl acetate. The organic layer was dried over sodium sulphate and concentrated under reduced pressure to afford the title compound as light yellow liquid (15.1 g, 93% yield). 1H-NMR (δ ppm, DMSO-D6, 400 MHz): δ 10.49 (s, 1H), 7.35 (d, J=6.2 Hz, 2H).
References:
US2011/118257,2011,A1 Location in patent:Page/Page column 100
461-96-1
478 suppliers
$6.00/10g

104197-13-9
245 suppliers
$6.00/1g
106-41-2
496 suppliers
$13.00/5g
2105-94-4
352 suppliers
$6.00/5g

104197-13-9
245 suppliers
$6.00/1g
372-18-9
422 suppliers
$9.00/1g

104197-13-9
245 suppliers
$6.00/1g
162101-25-9
335 suppliers
$6.00/1g

104197-13-9
245 suppliers
$6.00/1g