
4-bromo-1H-pyrrolo[2,3-c]pyridine synthesis
- Product Name:4-bromo-1H-pyrrolo[2,3-c]pyridine
- CAS Number:69872-17-9
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03
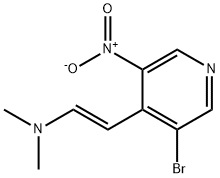
1258875-12-5
3 suppliers
inquiry
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
170 suppliers
$18.00/100mg
Yield:69872-17-9 73%
Reaction Conditions:
with iron;acetic acid for 0.75 h;Heating / reflux;
Steps:
94 4-bromo-1H-pyrrolo[2,3-c]pyridine (94c)
4-bromo-1H-pyrrolo[2,3-c]pyridine (94c) 2-(3-bromo-5-nitropyridin-4-yl)-N,N-dimethylethylenamine 94b (2.38 g, 8.78 mmol) and iron powder (~325 mesh, 2.45 g, 43.9 mmol) were stirred in acetic acid (25 mL). The red mixture was heated to reflux and turned grayish-green with a white precipitate. After refluxing for 45 min the reaction was cooled, diluted with EtOAc (150 mL), filtered through Celite and rinsed with additional EtOAc. The filtrate was slowly basified to pH 8 with saturated aqueous NaHCO3, and the aqueous layer was extracted with EtOAc. The combined organics were washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. Silica gel chromatography (eluding with 5% MeOH in EtOAc) afforded 94c (1.26 g, 73%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ 8.96 (br s, 1H), 8.74 (s, 1H), 8.37 (s, 1H),7.46 (m, 1H), 6.66 (m,1H).
References:
PFIZER INC US2005/90529, 2005, A1 Location in patent:Page/Page column 82-83
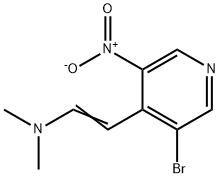
69872-16-8
0 suppliers
inquiry
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
170 suppliers
$18.00/100mg

31872-65-8
136 suppliers
$12.00/250mg
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
170 suppliers
$18.00/100mg

31872-63-6
177 suppliers
inquiry
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
170 suppliers
$18.00/100mg

69872-15-7
119 suppliers
inquiry
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
170 suppliers
$18.00/100mg