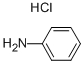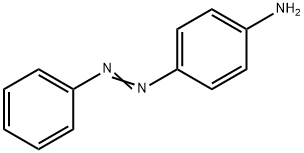
4-AMINOAZOBENZENE synthesis
- Product Name:4-AMINOAZOBENZENE
- CAS Number:60-09-3
- Molecular formula:C12H11N3
- Molecular Weight:197.24
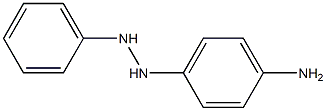
19689-52-2
0 suppliers
inquiry

60-09-3
207 suppliers
$17.67/1gm:
Yield:60-09-3 98 %
Reaction Conditions:
with oxygen in N,N-dimethyl-formamide at 80;
Steps:
35 Preparation of Azobenzene Analogue 2s
In an oxygen atmosphere, 1s (100mg, 0.5mmol) was dissolved in 2mL DMF and stirred at 80°C for 2h.30 mL of water was added to the reaction, the organic layer was washed with 30 mL of ethyl acetate, then washed with 30 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 98 mg of compound 2s with a yield of 98%.
References:
CN115368268,2022,A Location in patent:Paragraph 0175-0178
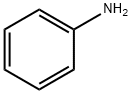
62-53-3
657 suppliers
$10.00/1g

60-09-3
207 suppliers
$17.67/1gm:
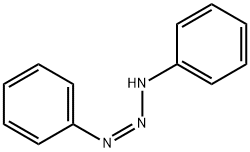
297766-64-4
0 suppliers
inquiry

60-09-3
207 suppliers
$17.67/1gm: