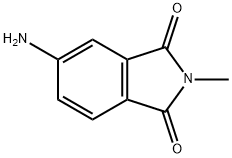
4-AMINO-N-METHYLPHTHALIMIDE synthesis
- Product Name:4-AMINO-N-METHYLPHTHALIMIDE
- CAS Number:2307-00-8
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
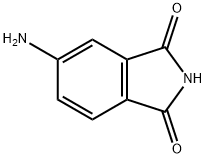
3676-85-5
224 suppliers
$45.00/1g

74-88-4
348 suppliers
$15.00/10g

2307-00-8
172 suppliers
$6.00/250mg
Yield: 92%
Reaction Conditions:
Stage #1:4-aminophthalimide with potassium hydroxide in N,N-dimethyl-formamide at 20; for 2 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
5-Amino-2-methyl-2,3-dihydro-1H-isoindole-1,3-dione (11a; ZHAWOC3444):
Potassiumhydroxide (0.35 g,6.17 mmol) was added to a solution of 4-aminophthalimide 10 (1.00 g, 6.17 mmol) in dimethylformamide(30 mL) and the mixture was stirred at ambient temperature for 2 h. Iodomethane (0.88 g, 6.17 mmol) wasadded and it was stirred for another 18 h at the same temperature. Water (50 mL) and ethyl acetate (50 mL)was added and the resulting phases were separated. The organic phase was washed with brine, driedover sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient:0-100% ethyl acetate in cyclohexane) afforded the title compound 11a as a yellow solid (1.00 g, 92% yield):1H-NMR (DMSO-d6): δ = 7.46 (d, J = 8.24 Hz, 1H), 6.90 (d, J = 2.00 Hz, 1H), 6.77 (dd, J = 8.24 Hz, 2.00 Hz,1H), 6.42 (br. s, 2H), 2.94 (s, 3H) ppm. 13C-NMR (DMSO-d6): δ = 168.87, 168.57, 155.27, 135.10, 125.17,117.33, 116.84, 107.42, 23.83 ppm. MS (m/z): 177 [M+ H]+.
References:
Fischer, Thomas;Riedl, Rainer [Molecules,2017,vol. 22,# 9]
41663-84-7
269 suppliers
$5.00/5g

2307-00-8
172 suppliers
$6.00/250mg
51832-31-6
31 suppliers
$65.00/100mg

2307-00-8
172 suppliers
$6.00/250mg

550-44-7
303 suppliers
$20.80/5g

2307-00-8
172 suppliers
$6.00/250mg
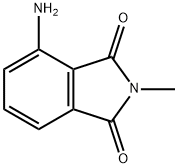
2257-85-4
52 suppliers
$60.00/500 mg
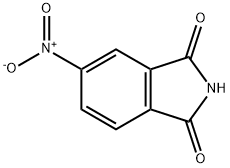
89-40-7
311 suppliers
$5.00/5g

2307-00-8
172 suppliers
$6.00/250mg