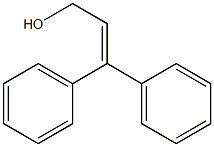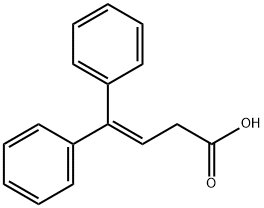
4,4-DIPHENYL-3-BUTENOIC ACID synthesis
- Product Name:4,4-DIPHENYL-3-BUTENOIC ACID
- CAS Number:7498-88-6
- Molecular formula:C16H14O2
- Molecular Weight:238.28
Yield:7498-88-6 96%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);acetic anhydride;nixantphos in toluene at 100; for 20 h;Inert atmosphere;Schlenk technique;Green chemistry;
Steps:
6 Example 6, 4,4-Diphenylbut-3-enoic acid
General procedure: In 10mL Schlenk reaction tube (Beijing Xin Weier Glass Instrument Co., Ltd., F891410 reaction tube, the capacity(Dibenzylidene indenone) dipalladium (Pd2 (dba) 3, 0.5 mol 2.3 mg), 4,5-bisdiphenylphosphine-9,9-dimethyl Xanthosine (2.0 mol%, 5.8 mg). The atmosphere in the tube was completely replaced with argon three times and then 1 mL of toluene, cinnamyl alcohol (0.50 mmol, 67 mg), formic acid (1.5 mmol, 69 mg) and acetic anhydride (1.5 mmol, 152 mg) were added under argon atmosphere. After the reaction tube was sealed, the reaction system was heated to 80 ° C under an oil bath and continuously stirred for 12 hoursIKA magnetic stirrer, RCT basic type, stirring speed 500 rev / min). After the reaction was completed, the system was cooled to room temperature. useThe reaction solution was diluted with ethyl acetate, and the diluted reaction solution was concentrated by rotary evaporation (Swiss Buchi Co.,BUCHI Rotavapor R-3). Concentrated residue through the column (Beijing Xin Weier Glass Instruments Co., Ltd., C383040C withSand storage ball column, 35/20, effective length: 500 mL, eluent petroleum ether: ethyl acetate = 5: 1 to 1:1)) to give the product by chromatography. The product was a white solid, total 72 mg, 89% yield.
References:
CN107337572,2017,A Location in patent:Paragraph 0041; 0042; 0043; 0044; 0045; 0046; 0047-0083
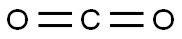
124-38-9
129 suppliers
$214.00/14L
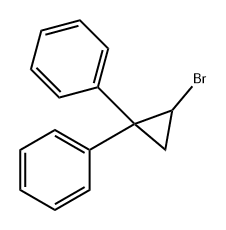
32812-52-5
0 suppliers
inquiry
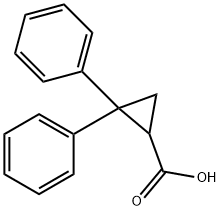
7150-12-1
39 suppliers
$140.00/0.25/ G

7498-88-6
16 suppliers
$28.60/5mg
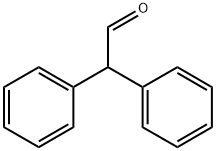
947-91-1
119 suppliers
$24.73/1gm:

141-82-2
756 suppliers
$5.00/25g

7498-88-6
16 suppliers
$28.60/5mg
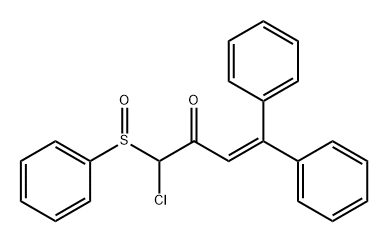
405506-58-3
0 suppliers
inquiry

7498-88-6
16 suppliers
$28.60/5mg
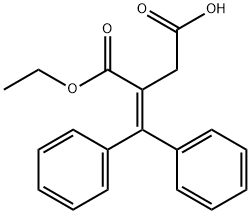
5438-22-2
16 suppliers
$45.00/10mg

7498-88-6
16 suppliers
$28.60/5mg