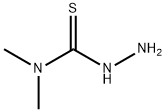
4,4-DIMETHYL-3-THIOSEMICARBAZIDE synthesis
- Product Name:4,4-DIMETHYL-3-THIOSEMICARBAZIDE
- CAS Number:6926-58-5
- Molecular formula:C3H9N3S
- Molecular Weight:119.19

16420-13-6
285 suppliers
$5.00/5g

6926-58-5
68 suppliers
$38.00/1g
Yield:-
Reaction Conditions:
with hydrazine in ethanol at 20;
Steps:
2
Example 2 - Synthesis of N-(l-[4-chlorophenyl]pyrrol-2-yl methylideneamino)- N',N',N",N"-tetramethyl guanidine.Dimethylthiocarbamoyl chloride (161 mg, 1.3 mmol) and hydrazine monohydrate (65 mg, 1.3 mmol) are mixed in ethanol (3 ml). The mixture is stirred at room temperature until the acid chloride is consumed. Methyl iodide is added (185 mg, 1.3 mmol) and the reaction mixture is stirred at room temperature. The solvent is removed in vacuo to give crude 4,4,S-trimethyl-isothiosemicarbazide hydroiodide, which is mixed with l-(4-chloro- phenyl)-pyrrole-2-carbaldehyde (206 mg, 1 mmol) in ethanol (5 ml) and heated at reflux. When the aldehyde is consumed the solvent is removed. The residue is suspended in dry toluene (5 ml) and a solution of dimethylamine (3 mmol) in dry toluene (1 ml) is added. The mixture is heated at reflux in a sealed vial until completion of the reaction. The solvent is removed and the solid residue is recrystallized to give the title product. The synthesis is illustrated in Fig. 5.
References:
ACTION PHARMA A/S WO2009/74157, 2009, A1 Location in patent:Page/Page column 74
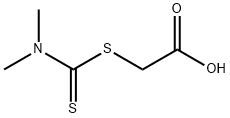
4007-01-6
37 suppliers
$33.40/250mg

6926-58-5
68 suppliers
$38.00/1g
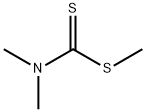
3735-92-0
22 suppliers
$498.76/5MG

6926-58-5
68 suppliers
$38.00/1g

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)