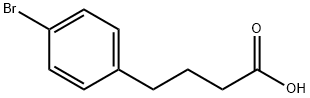
4-(4-BROMOPHENYL)BUTANOIC ACID synthesis
- Product Name:4-(4-BROMOPHENYL)BUTANOIC ACID
- CAS Number:35656-89-4
- Molecular formula:C10H11BrO2
- Molecular Weight:243.1
6340-79-0
156 suppliers
$5.00/250mg

35656-89-4
128 suppliers
$25.00/1g
Yield:35656-89-4 91.4%
Reaction Conditions:
with hydrogenchloride;mercury(II) chloride;zinc in water;toluene at 100; for 24 h;
Steps:
1 4-(4-bromophenyl)butanoic acid 5
Zinc (13.0 g, 200 mmol) and mercury chloride (1 .00 g, 480 mmol) were stirred with water (10 mL) and concentrated HCI (0.6 mL) for five minutes. The liquid was decanted off and toluene (20 mL), concentrated HCI (20 mL) and water (8 mL) were added consecutively. 4-(4-bromophenyl)-4-oxobutanoic acid 4 (2.55 g, 10.5 mmol) was added and heated under reflux at 100°C for 24 h adding HCI (1 mL) every 6 h. The reaction was allowed to cool to room temperature, filtered and the solvent removed from the organic layer to give a clear liquid which gave white crystals upon cooling. These were purified with silica gel chromatography (ethyl acetate : hexane, 1 :3) to yield 4-(4- bromophenyl)butanoic acid 5 (2.33 g, 9.63 mmol, 91 .4%). MS (ESI-QUADRUPOLE) m/z: calc. for CioHn BrO2: 241 .99, 243.99; Found: 244.0 (10), 243.0 (98), 242.0 (1 1 ), 241 .0 (100) (negative ions). HPLC: tr = 27.7 min. 1 H NMR (300 MHz, CDCI3) (δ/ppm) 7.40 (2H, d, J = 8.4 Hz, ArH), 7.06 (2H, d, J = 8.4 Hz, ArH), 2.63 (2H, t, J = 7.8 Hz, CH2), 2.36 (2H, t, J = 7.2 Hz, CH2), 1 .97, (2H, dt, J = 7.2,7.8 Hz, CH2), OH not observed.
References:
WO2016/119017,2016,A1 Location in patent:Page/Page column 22; 31

625-38-7
159 suppliers
$25.00/5g
5467-74-3
458 suppliers
$8.00/1g

35656-89-4
128 suppliers
$25.00/1g

108-86-1
506 suppliers
$10.00/5g

35656-89-4
128 suppliers
$25.00/1g
2294-43-1
36 suppliers
$256.00/1g

35656-89-4
128 suppliers
$25.00/1g
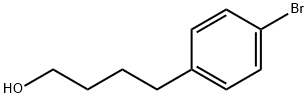
75906-36-4
36 suppliers
$110.00/0.5g

35656-89-4
128 suppliers
$25.00/1g