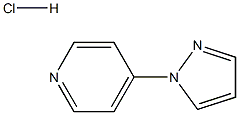
4-(1H-pyrazol-1-yl)pyridine hydrochloride synthesis
- Product Name:4-(1H-pyrazol-1-yl)pyridine hydrochloride
- CAS Number:1357589-48-0
- Molecular formula:C8H8ClN3
- Molecular Weight:181.6222
Yield:1357589-48-0 64%
Reaction Conditions:
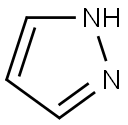
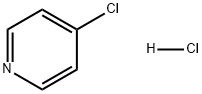
Stage #1: NH-pyrazole;4-chlorpyridine hydrochloride in acetonitrile; for 24 h;Inert atmosphere;Reflux;Large scale reaction;
Stage #2: with N-ethyl-N,N-diisopropylamine in acetonitrile at 20 - 25; for 0.5 h;Inert atmosphere;Large scale reaction;
Steps:
Synthesis of 4-(1H-pyrazol-1-yl)pyridine hydrochloride (10)
To acetonitrile (7 L) was added 4-chloropyridine hydrochloride (1.0 Kg, 6.67 mol) and 1H-pyrazole (1.13 Kg, 16.67 mol). After stirring at reflux for 24 hours under nitrogen, the resulting slurry was cooled to 20°C and diisopropylethylamine (861 g, 6.67 mol) was added at such a rate that internal temperature was lower than 25°C. After stirring at 20°C for 30mins, the product was filtered off and washed with acetonitrile (1 L) followed by ethyl acetate (4 L) at 20°C. The solid was dried at 50°C under vacuum and the product 4-(1H-pyrazol-1-yl)pyridine HCl 10 was isolated as an off-white solid (765 g, 64%); mp 195-196°C; IR (neat) (cm-1) 3260, 3110, 3050, 2650, 1630, 1520, 1400, 1280, 1020; 1H NMR (400MHz, DMSO-d6) δ 6.81 (1H, dd, J = 2.8 Hz & 1.4 Hz), 8.08 (1H, d, J = 1.4Hz), 8.43 (2H, dd, J = 7.4 Hz and 2.2 Hz), 8.96 (2H, dd, J = 7.4Hz and 2.2 Hz), 9.03 (1H, d, J = 2.8Hz); 13C NMR (100MHz, DMSO-d6) δ 111.5, 114.1, 130.5, 143.7, 145.5, 150.5. ESI-MS m/z 146 [M+H]+; Anal calcd for C8H8ClN3 (181.6): C, 52.90; H, 4.44; N, 23.14; Cl, 19.52; Found: C, 52.09; H, 4.54; N, 23.74; Cl, 19.18.
References:
Fussell, Steven J.;Luan, Amy;Peach, Philip;Scotney, Gemma [Tetrahedron Letters,2012,vol. 53,# 8,p. 948 - 951] Location in patent:supporting information; experimental part