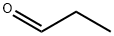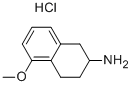
(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine hydrochloride synthesis
- Product Name:(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine hydrochloride
- CAS Number:3904-24-3
- Molecular formula:C14H22ClNO
- Molecular Weight:255.7836
101403-25-2
8 suppliers
inquiry

3904-24-3
18 suppliers
inquiry
Yield:3904-24-3 95%
Reaction Conditions:
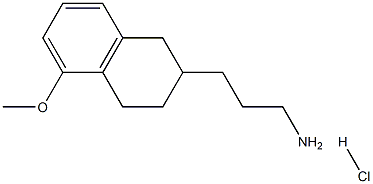
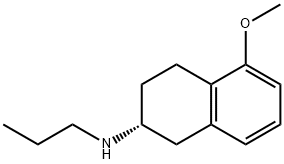
Stage #1: (R)-1,2,3,4-tetrahydro-5-methoxy-N-propyl-naphthalen-2-aminewith 1-dodecylthiol in ethyl acetate;Inert atmosphere;Reflux;
Stage #2: with 2,2'-azobis(isobutyronitrile) in ethyl acetate; for 0.583333 h;Inert atmosphere;Reflux;
Stage #3: with hydrogenchloride in water;ethyl acetate; for 0.0833333 h;Reflux;
Steps:
7.2
7.2. Preparation of (RS)-1 ,2,3,4-tetrahydro-5-methoxy-N-propyl- naphthalen-2- amine hydrocloride (lla); (R)-1 ,2,3,4-tetrahydro-5-methoxy-N-propyl-naphthalen-2-amine (III) (1 eq), obtained under example 7.1 ., is adjusted to a dilution of -9.5 vol. in EtOAc. 1-dodecanethiol is added (1.2eq) and the solution is heated to reflux under nitrogen atmosphere. A solution of AIBN (0.1 eq) in 0.5 vol. of EtOAc is dropwise added during 5 min. The solution is post- stirred under reflux for 30 min.Aqueous HCI 37% w:w (1.1 eq) is added dropwise under reflux for ~ 5min into the mixture obtained to precipitate the N-propyl MAT. HCI. The suspension is cooled down to 20°C (Tramp = -20°C/h). The suspension is post-stirred at 20°C for minimum 30 min and filtered. The cake is washed twice with EtOAc (2 x 1 vol.). The wet solid is dried under vacuum at 40°C giving an off white solid. Yield: 95.0% from (III)
References:
WO2011/161255,2011,A2 Location in patent:Page/Page column 33
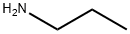
107-10-8
355 suppliers
$10.00/20mg
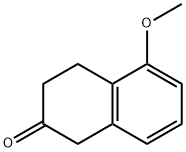
32940-15-1
308 suppliers
$13.43/250mgs:

3904-24-3
18 suppliers
inquiry
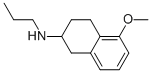
78598-91-1
14 suppliers
inquiry

3904-24-3
18 suppliers
inquiry