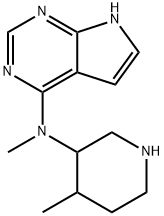
N-methyl-N-((3S,4S)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine synthesis
- Product Name:N-methyl-N-((3S,4S)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine
- CAS Number:384336-73-6
- Molecular formula:C13H19N5
- Molecular Weight:245.32
![N-(1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine](/CAS/20180527/GIF/384337-90-0.gif)
384337-90-0
8 suppliers
inquiry
![N-methyl-N-((3S,4S)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine](/StructureFile/ChemBookStructure22/GIF/CB5970946.gif)
384336-73-6
8 suppliers
inquiry
Yield:384336-73-6 90%
Reaction Conditions:
Stage #1: methyl(1-phenylmethyl-4-methylpiperidin-3-yl)-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-aminewith hydrogenchloride in ethanol;water;
Stage #2: with hydrogen;palladium hydroxide on carbon in ethanol;water at 20; under 2585.81 Torr; for 48 h;
Steps:
1.C
To the product from Method B (0.7 grams, 2.19 mmol) dissolved in 15 mL of ethanol was added 1.5 mL of 2 N hydrochloric acid and the reaction mixture degassed by nitrogen purge. To the reaction mixture was then added 0.5 grams of 20% palladium hydroxide on carbon (50% water) (Aldrich) and the resulting mixture shaken (Parr-Shaker) under a 50 psi atmosphere of hydrogen at room temperature for 2 days. The Celite filtered reaction mixture was concentrated to dryness in vacuo and the residue purified by flash chromatography (silica ; 5% methanol in dichoromethane) affording 0.48 grams (90%) of the title compound.
References:
WO2005/60972,2005,A2 Location in patent:Page/Page column 38
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
833 suppliers
$6.00/1g
![N-methyl-N-((3S,4S)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine](/StructureFile/ChemBookStructure22/GIF/CB5970946.gif)
384336-73-6
8 suppliers
inquiry
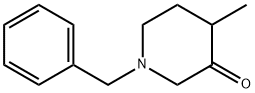
32018-96-5
124 suppliers
$60.00/1g
![N-methyl-N-((3S,4S)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine](/StructureFile/ChemBookStructure22/GIF/CB5970946.gif)
384336-73-6
8 suppliers
inquiry
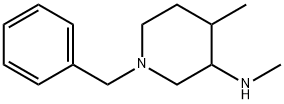
384338-23-2
79 suppliers
$75.00/1g
![N-methyl-N-((3S,4S)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine](/StructureFile/ChemBookStructure22/GIF/CB5970946.gif)
384336-73-6
8 suppliers
inquiry