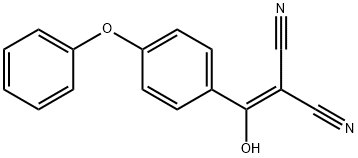
Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]- synthesis
- Product Name:Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]-
- CAS Number:330792-68-2
- Molecular formula:C16H10N2O2
- Molecular Weight:262.26
Yield: 96%
Reaction Conditions:
Stage #1:4-Phenoxybenzoic acid with thionyl chloride for 1 h;Heating / reflux;
Stage #2:malononitrile with N-ethyl-N,N-diisopropylamine in tetrahydrofuran;toluene at -10 - 20;
Steps:
Briefly, 4-phenoxybenzoic acid (48 g) is added to thionyl chloride (100 mL) and heated under gentle reflux for 1 hour. Thionyl chloride was removed by distillation, the residual oil was dissolved in toluene and volatile material removed at 80° C./20 mbar. The resulting acid chloride was dissolved in toluene (200 mL) and tetrahydrofuran (35 mL). Malononitrile (14.8 g) was added and the solution and stirred at -10° C. while adding diisopropylethylethylamine (57.9 g) in toluene (150 mL), while maintaining the temperature below 0° C. After 1 hour at 0° C., the mixture was stirred at 20° C. overnight. Amine hydrochloride is removed by filtration and the filtrate evaporated in vacuo. The residue was taken up in ethyl acetate and washed with 1.25 M sulphuric acid, then with brine and dried over sodium sulfate. Evaporation of the solvents gave a semisolid residue which was treated with a portion of ethyl acetate to give 4.1 g of 1,1-dicyano-2-hydroxy-2-(4-phenoxyphenyl)ethene as a white solid (m.p. 160-162° C.). The filtrate on evaporation gave 56.58 (96%) of 1,1-dicyano-2-hydroxy-2-(4-phenoxyphenyl)ethene as a grey-brown solid, which was sufficiently pure for further use.
References:
PHARMACYCLICS, INC. US2008/214501, 2008, A1 Location in patent:Page/Page column 11
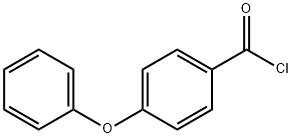
1623-95-6
45 suppliers
$215.61/1gm:

109-77-3
429 suppliers
$10.00/5g
![Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]-](/CAS/20150408/GIF/330792-68-2.gif)
330792-68-2
55 suppliers
inquiry
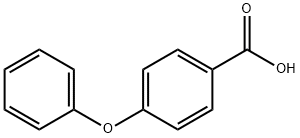
2215-77-2
392 suppliers
$8.00/5g
![Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]-](/CAS/20150408/GIF/330792-68-2.gif)
330792-68-2
55 suppliers
inquiry
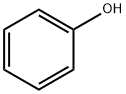
108-95-2
735 suppliers
$14.00/25g
![Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]-](/CAS/20150408/GIF/330792-68-2.gif)
330792-68-2
55 suppliers
inquiry
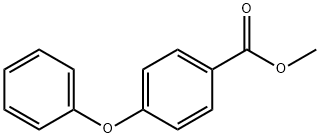
21218-94-0
42 suppliers
$60.00/1g
![Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]-](/CAS/20150408/GIF/330792-68-2.gif)
330792-68-2
55 suppliers
inquiry