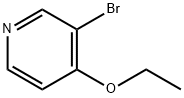
3-BROMO-4-ETHOXYPYRIDINE synthesis
- Product Name:3-BROMO-4-ETHOXYPYRIDINE
- CAS Number:3522-97-2
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
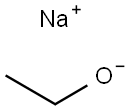
36953-42-1
266 suppliers
$6.00/1g
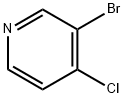
141-52-6
462 suppliers
$10.00/5g

3522-97-2
24 suppliers
$123.00/250mg
Yield:3522-97-2 84%
Reaction Conditions:
in tetrahydrofuran;ethanol at 55; for 6 h;
Steps:
415.1 Step 1
To a solution of compound (VII-48) (300 mg, 1.56 mmol) in THF (5.0 mL) was added sodium ethoxide (20%ethanol solution, 1.8 mL, 4.6 mmol), and the mixture was stirred at 55°C for 6 hr. The reaction mixture was cooled toroom temperature, water was added, and the mixture was extracted with ethyl acetate. The organic layer was washedsuccessively with water and saturated brine, dried over anhydrous sodium sulfate, and filtered. The solvent was evaporatedunder reduced pressure, and the residue was purified by silica gel column chromatography (NH silica gel, nhexane:ethyl acetate = 95:5 → 50:50) to give compound (VII-55) (yield 264 mg, 84%) as a yellow oil
References:
EP3351533,2018,A1 Location in patent:Paragraph 0962; 0963