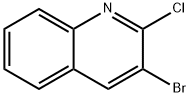
3-BROMO-2-CHLOROQUINOLINE synthesis
- Product Name:3-BROMO-2-CHLOROQUINOLINE
- CAS Number:101870-60-4
- Molecular formula:C9H5BrClN
- Molecular Weight:242.5
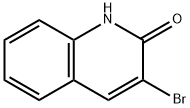
939-16-2
69 suppliers
$90.00/50mg

101870-60-4
68 suppliers
$29.00/250mg
Yield: 89%
Reaction Conditions:
with trichlorophosphate in acetonitrile at 20; for 3 h;Reflux;
Steps:
5.3 3-Bromo-2-chloroquinoline (16)
To a stirred suspension of 3-bromo-1H-quinolin-2-one (15) (4.16 g, 18.57 mmol) in acetonitrile (30 mL) phosphorus oxychloride (3.42 g, 22.28 mmol) was added dropwise at room temperature.
The reaction mixture was refluxed for 3 h.
After cooling to room temperature it was poured on crushed ice (70 mL) and the pH was adjusted to 9 with potassium carbonate solution (10%, 40 mL).
The resulting precipitate was filtered off, washed with water and dried in vacuo.
The title compound was obtained as a white solid (4.00 g, 89%), mp 101-103 °C (lit.,
34
mp 96-98 °C); 1H NMR (DMSO-d6, 400 MHz) δ 8.95 (s, 1H), 8.03 (d, 1H, J=8.0 Hz), 7.98 (d, 1H, J=8.8 Hz), 7.90-7.84 (m, 1H), 7.74-7.69 (m, 1H); 13C NMR (DMSO-d6, 400 MHz) δ 148.1, 145.4, 142.1, 131.3, 128.1, 127.8, 127.6, 127.2, 115.6; C9H5BrClN (242.50); LCMS (ESI+) m/z 242, 244 [M+H]+; Anal. Calcd for C9H5BrClN (242.50) C, 44.58; H, 2.08; N, 5.78. Found: C, 44.46; H, 2.07; N, 5.79.
References:
Bogányi, Borbála;Kámán, Judit [Tetrahedron,2013,vol. 69,# 45,p. 9512 - 9519]
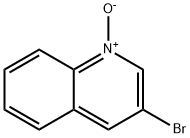
22615-00-5
38 suppliers
$45.00/100mg

101870-60-4
68 suppliers
$29.00/250mg

939-16-2
69 suppliers
$90.00/50mg
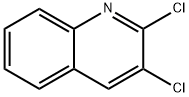
613-18-3
43 suppliers
$70.00/250 mg

101870-60-4
68 suppliers
$29.00/250mg

5332-24-1
363 suppliers
$10.00/5g

101870-60-4
68 suppliers
$29.00/250mg