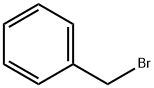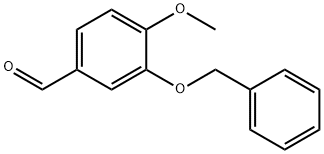
3-Benzyloxy-4-methoxybenzaldehyde synthesis
- Product Name:3-Benzyloxy-4-methoxybenzaldehyde
- CAS Number:6346-05-0
- Molecular formula:C15H14O3
- Molecular Weight:242.27
Yield:6346-05-0 100%
Reaction Conditions:
with potassium carbonate in methanol; for 4 h;Reflux;
Steps:
3-benzyloxy-4-methoxybenzaldehyde 5:
The mixture of aldehyde 4 (10 g, 65.8 mmol), benzyl bromide (9.38 mL, 79.0 mmol), and potassium carbonate (10.9 g, 79.0 mmol) in methanol (40 mL) was refluxed for 4 h. The reaction mixture was filtered and the filtrate was evaporated under reduced pressure. Chloroform (80 mL) was added to the residue, and the solution was washed with water (2 × 30 mL), the organic layer was filtered using a Biotage ISOLUTE phase separator and then evaporated to afford a white powder (15 g, yield: 100%). 1H NMR (CDCl3, 400 MHz): δ = 9.84 (1H, s, CHO), 7.50-7.47 (4H, m, Ph-H), 7.42-7.32 (3H, m, Ph-H), 7.02 (1H, d, J = 8.8 Hz, Ph-H), 5.21 (2H, s, OCH2Ph), 3.98 (3H, s, OCH3) ppm. 13C NMR (CDCl3, 100 MHz): δ = 190.9, 155.1, 148.7, 136.3, 130.0, 128.7, 128.1, 127.5, 126.9, 111.4, 110.8, 70.9, 56.2 ppm. ESI-MS: calc. for C15H15O3 [M+H]+: 243.1, found: 243.2. HPLC purity: 99.1% (254 nm), tR: 6.82 min; 97.9% (220 nm), tR: 6.82 min.
References:
Shen, Li;Sun, Dianqing [Tetrahedron Letters,2011,vol. 52,# 35,p. 4570 - 4574] Location in patent:supporting information; experimental part
1860-60-2
56 suppliers
$137.00/1g

6346-05-0
212 suppliers
$7.00/1g
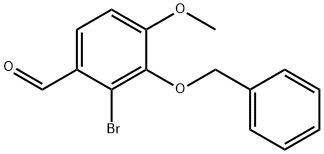
38849-38-6
5 suppliers
inquiry

6346-05-0
212 suppliers
$7.00/1g

621-59-0
625 suppliers
$9.00/1g

6346-05-0
212 suppliers
$7.00/1g