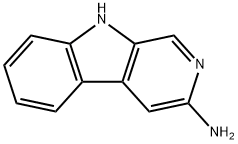
3-aminonorharman synthesis
- Product Name:3-aminonorharman
- CAS Number:73834-77-2
- Molecular formula:C11H9N3
- Molecular Weight:183.21
![9H-PYRIDO[3,4-B]INDOLE-3-CARBOXYLIC ACID HYDRAZIDE](/CAS/20180703/GIF/73834-74-9.gif)
73834-74-9
1 suppliers
inquiry

73834-77-2
49 suppliers
$75.00/10mg
Yield:73834-77-2 78%
Reaction Conditions:
Stage #1: 9H-pyrido<3,4-b>indole-3-carboxylic acid hydrazidewith hydrogenchloride;NaNO2 in lithium hydroxide monohydrate at 0;
Stage #2: with glacial acetic acid in lithium hydroxide monohydrate at 110; pH=8;
Steps:
1.6 (6) Synthesis of compounds 6a-6f:
Take 6a as an example, weigh 5a (10mmol) and place it in a 1L beaker, add water (200ml) and stir, then add concentrated hydrochloric acid (20ml), aqueous sodium nitrite (23mmol) to the solution. Stir for 10-30 min, keep the reaction temperature below 0 °C, and monitor the reaction by TLC. After the reaction was completed, the pH of the solution was adjusted to 8 with NaHCO3 and NaOH, and the solid was obtained by suction filtration (the solid was washed with a large amount of clean water, and could be directly used in the next step without drying). The product of the previous step (10 mmol) was added to a mixed solution of water (150 ml) and glacial acetic acid (150 ml), and refluxed at 110 °C for 2-4 h, (TLC monitoring reaction) after the reaction is completed, the solution is cooled, concentrated under reduced pressure to remove the solvent. The pH was adjusted to 8 with NaHCO3, the solution was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 6a with a yield of 78%.
References:
CN114621225,2022,A Location in patent:Paragraph 0088; 0091; 0097; 0098
![9H-Pyrido[3,4-b]indole-3-carbonyl azide](/CAS/20211123/GIF/73834-75-0.gif)
73834-75-0
0 suppliers
inquiry

73834-77-2
49 suppliers
$75.00/10mg
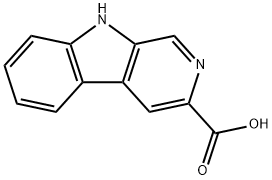
74214-63-4
6 suppliers
inquiry

73834-77-2
49 suppliers
$75.00/10mg
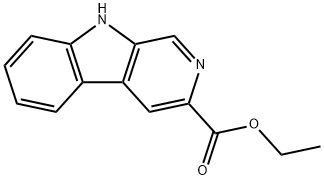
74214-62-3
55 suppliers
$45.00/100mg

73834-77-2
49 suppliers
$75.00/10mg
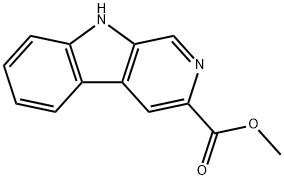
69954-48-9
14 suppliers
inquiry

73834-77-2
49 suppliers
$75.00/10mg