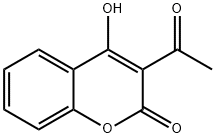
3-acetyl-4-hydroxy-2-benzopyrone synthesis
- Product Name:3-acetyl-4-hydroxy-2-benzopyrone
- CAS Number:2555-37-5
- Molecular formula:C11H8O4
- Molecular Weight:204.18
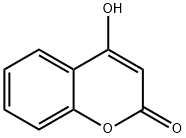
1076-38-6
539 suppliers
$24.00/100g
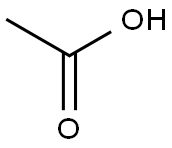
64-19-7
1558 suppliers
$10.00/25ML

2555-37-5
31 suppliers
$45.00/100mg
Yield:2555-37-5 92%
Reaction Conditions:
with trichlorophosphate for 1 h;Schlenk technique;Inert atmosphere;Reflux;
Steps:
General procedure for synthesis of 3-acetyl-4-hydroxycoumarin (1):
In a round bottom flask, 3 g (18.6 mmol) of 4-hydroxycoumarin, 16 mL of acetic acid (27.9 mmol) (solvent and reagent at a time) and 5.6 mL (60 mmol) of phosphorus oxychloride are added. The reaction was refluxed under continuous stirring for 1 h. Control of the evolution of the reaction by thin layer chromatography (TLC) (eluent: hexane-ethanol 80:20) revealed the formation of a single compound. At the end of the reaction, the product was filtered, recrystallized from ethanol, dried and recovered (Scheme-I). Yield: 92 %; m.p.: 225 °C; FT-IR (KBr, νmax, cm-1): 3185 (O-H); 1700 (CO lactone); 1H NMR (400 MHz, DMSO-d6) ppm δ: 2.45 (s, 3H, CO-CH3); 7.15-7.92 (m, 4H, Harom), 17.72 (s, OH). 13C NMR (100 MHz, DMSO-d6) in ppm δ: 32.36 (H3C-CO); 102.32 (aryl-C=C-OH); 115.91, 122.98, 125.66 (Carom) 132.95 (O-Carom); 157.54 (CO-O); 162.92 (C=C-OH); 176.70 (H3C-CO). Elemental analysis % calcd. (found) for C11H8O4: C, 64.707 (64.60); H, 3.949 (4.09); N, 8.123 (8.07).
References:
Mnasri, Aziza;Chakchouk-Mtibaa;Al-Ayed, Abdullah Sulaiman;Jemmali, Mosbah;Mellouli;Bilel, Hallouma;Hamdi, Naceur [Asian Journal of Chemistry,2019,vol. 31,# 7,p. 1609 - 1616]

1076-38-6
539 suppliers
$24.00/100g

75-36-5
577 suppliers
$17.92/100G

2555-37-5
31 suppliers
$45.00/100mg

1076-38-6
539 suppliers
$24.00/100g

2555-37-5
31 suppliers
$45.00/100mg
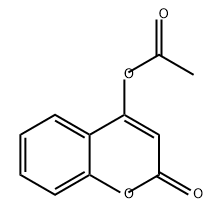
15059-36-6
0 suppliers
inquiry

2555-37-5
31 suppliers
$45.00/100mg
![Butanoic acid, 2-[[2-(acetyloxy)phenyl]hydroxymethylene]-3-oxo-, ethyl ester](/CAS/20210305/GIF/770719-72-7.gif)
770719-72-7
0 suppliers
inquiry

2555-37-5
31 suppliers
$45.00/100mg
