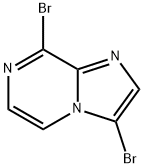
3,8-Dibromoimidazo[1,2-a]pyrazine synthesis
- Product Name:3,8-Dibromoimidazo[1,2-a]pyrazine
- CAS Number:936361-36-3
- Molecular formula:C6H3Br2N3
- Molecular Weight:276.92
![IMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/274-79-3.gif)
274-79-3
175 suppliers
$6.00/100mg
![3,8-Dibromoimidazo[1,2-a]pyrazine](/CAS/20150408/GIF/936361-36-3.gif)
936361-36-3
10 suppliers
inquiry
Yield: 97%
Reaction Conditions:
with bromine;acetic acid at 20; for 72 h;
Steps:
Synthesis
To a solution of imidazo[1,2-a]pyrazine (intermediate C) (2.8 g, 23.5 mmol) in AcOH (acetic acid) (200 ml) was added bromine (6 ml, 5 eq.) via addition funnel, with the reaction flask protected from light. After addition, the flask was sealed. The mixture was stirred at rt for 24 hr., 6 ml (5 eq.) of additional Br2 was added to the reaction mixture, which was further stirred at rt for 48 hr. The mixture was evaporated to remove Br2 and acetic acid, and the residue was dissolved in 10% IPA/DCM, washed with sat. Na2CO3 (300 ml). The organics were combined, dried and concentrated to afford 5.9 g (yield 97%) of 3,5-dibromo-imidazo[1,2-a]pyrazine. 1H-NMR (400 MHz, DMSO-d6) δ 9.05 (s, 1H), 8.09 (s, 1H), 7.99 (s, 1H). MS m/z 278 [M++1].
References:
Sugen, Inc. US2004/220189, 2004, A1 Location in patent:Page 21-22
![8-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/69214-33-1.gif)
69214-33-1
146 suppliers
$18.00/250mg
![3-BROMO-8-CHLOROIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/143591-61-1.gif)
143591-61-1
128 suppliers
$14.00/250mg
![3,8-Dibromoimidazo[1,2-a]pyrazine](/CAS/20150408/GIF/936361-36-3.gif)
936361-36-3
10 suppliers
inquiry