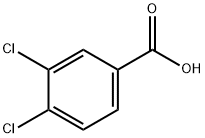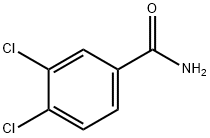
3,4-DICHLOROBENZAMIDE synthesis
- Product Name:3,4-DICHLOROBENZAMIDE
- CAS Number:2670-38-4
- Molecular formula:C7H5Cl2NO
- Molecular Weight:190.03
Yield: 84.44%
Reaction Conditions:
Stage #1:oxalyl dichloride;3,4-dichlorbenzoic acid in N,N-dimethyl-formamide at 0 - 25; for 12 h;
Stage #2: with ammonium hydroxide in dichloromethane at 0; for 1 h;
Steps:
[000859] Preparation of 3,4-dichlorobenzamide (Step 1 in Scheme C-13)
To a solution of 3,4-dichlorobenzoic acid (2 g,10.47 mmol,1 eq) in oxalyl dichloride (6.65 g, 52.35 mmol, 4.58 mL, 5 eq) was added DMF (38.26 mg, 523.53 miho, 40.28 pL, 0.05 eq) dropwise at 0 °C. The mixture was stirred at 25°C for 12 hours. The solvent was removed under reduced pressure. The residue was diluted with DCM (2 mL) and added to NH3 H20 (7.34 g, 52.35 mmol, 8.07 mL, 25% purity, 5 eq) dropwise at 0°C. Then the mixture was stirred at 0°C for 1Hour. TLC showed the reaction was complete. There was some white solid formed. After filtered, the solid was washed with H20 (10 mL x 3) and collected. Compound 3,4-dichlorobenzamide (1.7 g, 8.95 mmol, 85.44% yield) was obtained as white solid. 1H NMR (DMSO-d6, 400 MHz) d 8.15 (s,1H), 8.09 (d, J= 2.4 Hz,1H), 7.84 (dd, J= 8.4 Hz, 2.0 Hz,1H), 7.74 (d, J= 8.4 Hz,1H), 7.61 (s,1H).
References:
ACURX PHARMACEUTICALS, LLC;YU, Xiang Y.;XING, Li H.;WANG, Minghua;MCCOMAS, Casey;SILVERMAN, Michael;SOLL, Richard WO2020/132174, 2020, A1 Location in patent:Paragraph 000859

6287-38-3
254 suppliers
$8.00/10g

2670-38-4
83 suppliers
$44.00/1g
5331-92-0
40 suppliers
$26.10/1g

2670-38-4
83 suppliers
$44.00/1g
6574-99-8
333 suppliers
$10.00/1g

2670-38-4
83 suppliers
$44.00/1g
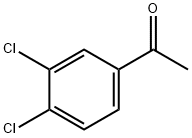
2642-63-9
189 suppliers
$6.00/5g

2670-38-4
83 suppliers
$44.00/1g