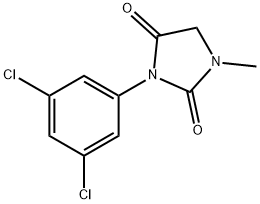
3-(3,5-Dichlorophenyl)-1-methylhydantoin synthesis
- Product Name:3-(3,5-Dichlorophenyl)-1-methylhydantoin
- CAS Number:27387-90-2
- Molecular formula:C10H8Cl2N2O2
- Molecular Weight:259.09
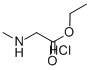
52605-49-9
348 suppliers
$6.00/10g
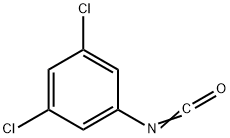
34893-92-0
217 suppliers
$21.00/1g

27387-90-2
44 suppliers
inquiry
Yield:27387-90-2 90%
Reaction Conditions:
Stage #1: sarcosine ethyl ester hydrochloridewith triethylamine in dichloromethane at 20; for 1.5 - 2 h;
Stage #2: 3,4-dichlorophenyl isocyanate in dichloromethane at 10 - 25; for 12.5 - 15 h;
Steps:
1 3-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione
Triethylamine (0.78 kg, 7.75 mol) was added in 15-30 minutes with stirring to a thin suspension of sarcosine ethylene hydrochloride (1.00 kg, 6.51 mol) in dichloromethane (6.00 L). After stirring at room temperature for 1.5-2.0 hours, the mixture was filtered to remove the resulting triethylamine hydrochloride salt. The salt cake was washed with dichloromethane (2.00 L). The filtrate was cooled to 0-5° C; A solution of 3,5-dichlorophenyl isocyanate (1.47 kg, 7.81 mol) in dichloromethane was prepared at 20-25° C. The solution was added to the above cooled filtrate slowly in 30-60 minutes. The temperature was maintained below 10° C. during the addition. After the addition, the mixture was stirred at 20-25° C. for 12-14 hours. The completeness of the reaction was followed by HPLC. Upon reaction completion, TBME (16.00 L) was added in one portion. The resulting suspension was stirred at 20-25° C. for 2-3 hours and was then filtered. The filter cake was washed with TBME (4.50 L) and dried at maximum 40° C. to a constant weight. A suspension of the above filter cake in water (17.0 L, 10 L/kg input) was prepared and stirred at 20-25° C. for at least 16 hours. The suspension was filtered and the filter cake was washed with water (3*1.36 L) and dried at maximum 40° C. to a constant weight to a constant weight. 3-(3,5-dichlorophenyl)-1-methylimidazolidine-2,4-dione (1.52 kg, 90%) was obtained as a white crystalline solid. mp=202-204° C. 1H NMR (DMSO-d6): 7.66 (1H, m), 7.51 (2H, m), 4.10 (2H, s), 3.35 (3H, s). 13C NMR (DMSO-d6): 8 Carbons (169.30, 155.00, 134.98, 134.15, 127.59, 125.30, 51.75, 29.79). Anal. Calcd for C10H8Cl2N2O2: C, 46.35; H, 3.1 1; N, 10.81; Cl, 27.36. Found: C, 46.43; H, 2.9; N, 10.73; Cl, 27.33.
References:
US2006/74099,2006,A1 Location in patent:Page/Page column 24
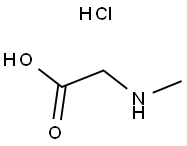
637-96-7
117 suppliers
$45.00/250mg

34893-92-0
217 suppliers
$21.00/1g

27387-90-2
44 suppliers
inquiry
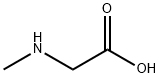
107-97-1
584 suppliers
$5.00/100mg

34893-92-0
217 suppliers
$21.00/1g

27387-90-2
44 suppliers
inquiry
